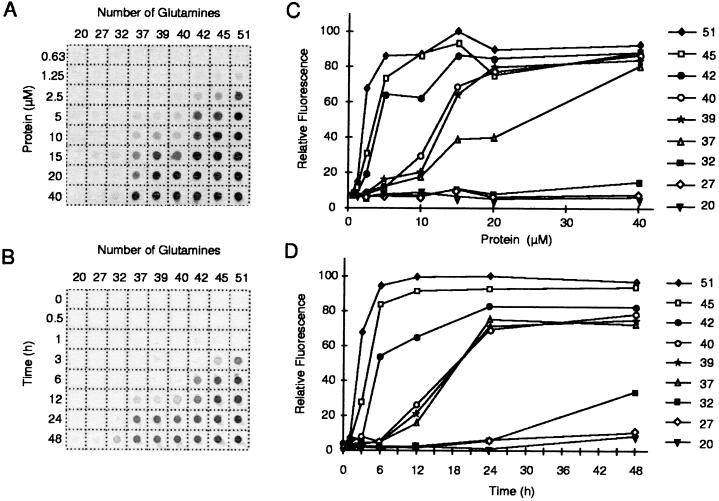
Figure 4.
(A) Concentration dependence of HDex1p aggregation. GST-HDex1 proteins with poly(Q) tracts of different lengths were incubated at the indicated concentrations with trypsin for 24 h at 37°C. Aliquots (200 ng) of each protein were then diluted into 0.2 ml of 2% SDS/50 mM DTT, boiled for 3 min, and filtered through a cellulose acetate membrane. Captured aggregates were detected by incubation with anti-AG51 serum (1:1,000), followed by incubation with alkaline phosphatase-conjugated anti-rabbit secondary antibody and the fluorescent substrate AttoPhos. (B) Time course of HDex1p aggregation. The various GST-HDex1 proteins were incubated at a concentration of 20 μM with trypsin. At the indicated times, aliquots (200 ng) of each protein were removed and analyzed by the filter retardation assay as in A. (C and D) Quantitative analysis of the dot-blot results shown in A and B, respectively. The relative amount of aggregate for each sample was quantitated on a Fuji-Imager (LAS 2000). For each experiment, the dot with the highest signal intensity was arbitrarily set as 100. The data reported are representative for three independent experiments using, in part, different GST-HDex1p preparations.