Abstract
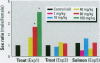
Fish sexual development is sensitive to exogenous hormone manipulation, and salmonids have been used extensively as environmental sentinels and models for biomedical research. We simulated maternal transfer of contaminants by microinjecting rainbow trout (Oncorhynchus mykiss) and chinook salmon (Oncorhynchus tshawytscha) embryos. Fish were reared for 6 months and sexed, and gonads were removed for histology and measurement of in vitro steroid production. Analysis of fat samples showed that dichlorodiphenylethylene (DDE) levels, o, p'M-DDE and p,o, p'-DDE isomers, were elevated 6 months after treatment. A preliminary study showed an increased ratio of males to females after treatment with 80 mg/kg and 160 mg/kg of the xenoestrogen o,o, p'-DDE. One fish treated with 160 mg/kg o,o, p'-DDE had gonads with cells typical of both males and females. A follow-up study, using more fish and excluding the highly toxic 160 mg/kg o,o, p'-DDE dose, showed no effect on sex ratio or gonadal histology. Embryonic exposure of monosex male trout, monosex female trout, and mixed sex salmon to o, o, p'-DDE, p,o, p'-DDE, mixtures of DDE isomers, and octylphenol failed to alter sexual development. We observed no treatment-dependent changes in in vitro gonadal steroid production in any experiments. Trout exposed in ovo and reared to maturity spawned successfully. These results suggest that mortality attributable to the xenoestrogens o,o, p'-DDE, chlordecone, and octylphenol, and the antiandrogen p,o, p'-DDE, is likely to occur before the appearance of subtle changes in sexual development. Because trout appeared to be sensitive to endocrine disruption, we cannot dismiss the threat of heavily contaminated sites or complex mixtures to normal sexual development of salmonids or other aquatic organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg U. G., Lipworth L., Titus-Ernstoff L., Hsieh C. C., Hanberg A., Baron J., Trichopoulos D., Adami H. O. Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: an assessment of the biological and epidemiological evidence. Crit Rev Toxicol. 1995;25(6):463–531. doi: 10.3109/10408449509017924. [DOI] [PubMed] [Google Scholar]
- Allen-Gil S. M., Gubala C. P., Wilson R., Landers D. H., Wade T. L., Sericano J. L., Curtis L. R. Organochlorine pesticides and polychlorinated biphenyls (PCBs) in sediments and biota from four US Arctic lakes. Arch Environ Contam Toxicol. 1997 Nov;33(4):378–387. doi: 10.1007/s002449900267. [DOI] [PubMed] [Google Scholar]
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara R. H., Born G. S., Christian J. E. Radiotracer distribution and excretion study of chlorophenothane in rats. J Pharm Sci. 1972 Dec;61(12):1912–1916. [PubMed] [Google Scholar]
- Black J. J., Maccubbin A. E., Schiffert M. A reliable, efficient, microinjection apparatus and methodology for the in vivo exposure of rainbow trout and salmon embryos to chemical carcinogens. J Natl Cancer Inst. 1985 Dec;75(6):1123–1128. [PubMed] [Google Scholar]
- Fitzpatrick M. S., Pereira C. B., Schreck C. B. In vitro steroid secretion during early development of mono-sex rainbow trout: sex differences, onset of pituitary control, and effects of dietary steroid treatment. Gen Comp Endocrinol. 1993 Aug;91(2):199–215. doi: 10.1006/gcen.1993.1119. [DOI] [PubMed] [Google Scholar]
- Gladen B. C., Rogan W. J., Hardy P., Thullen J., Tingelstad J., Tully M. Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J Pediatr. 1988 Dec;113(6):991–995. doi: 10.1016/s0022-3476(88)80569-9. [DOI] [PubMed] [Google Scholar]
- Golden R. J., Noller K. L., Titus-Ernstoff L., Kaufman R. H., Mittendorf R., Stillman R., Reese E. A. Environmental endocrine modulators and human health: an assessment of the biological evidence. Crit Rev Toxicol. 1998 Mar;28(2):109–227. doi: 10.1080/10408449891344191. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Guiney P. D., Melancon M. J., Jr, Lech J. J., Peterson R. E. Effects of egg and sperm maturation and spawning on the distribution and elimination of a polychlorinated biphenyl in rainbow trout (Salmo gairdneri). Toxicol Appl Pharmacol. 1979 Feb;47(2):261–272. doi: 10.1016/0041-008x(79)90320-x. [DOI] [PubMed] [Google Scholar]
- Guiney P. D., Peterson R. E. Distribution and elimination of a polychlorinated biphenyl after acute dietary exposure in yellow perch and rainbow trout. Arch Environ Contam Toxicol. 1980;9(6):667–674. doi: 10.1007/BF01055542. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18(2):415–424. [PubMed] [Google Scholar]
- Johnson A., Norton D., Yake B. Persistence of DDT in the Yakima River drainage, Washington. Arch Environ Contam Toxicol. 1988 May;17(3):289–297. doi: 10.1007/BF01055165. [DOI] [PubMed] [Google Scholar]
- Le Dréan Y., Kern L., Pakdel F., Valotaire Y. Rainbow trout estrogen receptor presents an equal specificity but a differential sensitivity for estrogens than human estrogen receptor. Mol Cell Endocrinol. 1995 Mar;109(1):27–35. doi: 10.1016/0303-7207(95)03482-m. [DOI] [PubMed] [Google Scholar]
- Le Dréan Y., Lazennec G., Kern L., Saligaut D., Pakdel F., Valotaire Y. Characterization of an estrogen-responsive element implicated in regulation of the rainbow trout estrogen receptor gene. J Mol Endocrinol. 1995 Aug;15(1):37–47. doi: 10.1677/jme.0.0150037. [DOI] [PubMed] [Google Scholar]
- Lustig R. H., Mobbs C. V., Bradlow H. L., McEwen B. S., Pfaff D. W. Differential effects of estradiol and 16 alpha-hydroxyestrone on pituitary and preoptic estrogen receptor regulation. Endocrinology. 1989 Nov;125(5):2701–2709. doi: 10.1210/endo-125-5-2701. [DOI] [PubMed] [Google Scholar]
- Mommsen T. P., Lazier C. B. Stimulation of estrogen receptor accumulation by estradiol in primary cultures of salmon hepatocytes. FEBS Lett. 1986 Jan 20;195(1-2):269–271. doi: 10.1016/0014-5793(86)80174-0. [DOI] [PubMed] [Google Scholar]
- Nimrod A. C., Benson W. H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996 May;26(3):335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Pajor A. M., Stegeman J. J., Thomas P., Woodin B. R. Feminization of the hepatic microsomal cytochrome P-450 system in brook trout by estradiol, testosterone, and pituitary factors. J Exp Zool. 1990 Jan;253(1):51–60. doi: 10.1002/jez.1402530108. [DOI] [PubMed] [Google Scholar]
- Pakdel F., Le Guellec C., Vaillant C., Le Roux M. G., Valotaire Y. Identification and estrogen induction of two estrogen receptors (ER) messenger ribonucleic acids in the rainbow trout liver: sequence homology with other ERs. Mol Endocrinol. 1989 Jan;3(1):44–51. doi: 10.1210/mend-3-1-44. [DOI] [PubMed] [Google Scholar]
- Podreka S., Georges A., Maher B., Limpus C. J. The environmental contaminant DDE fails to influence the outcome of sexual differentiation in the marine turtle Chelonia mydas. Environ Health Perspect. 1998 Apr;106(4):185–188. doi: 10.1289/ehp.98106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder A. L., Foley G. L., Nichols D. K., Hansen L. G., Wikoff B., Faeh S., Eisold J., Wheeler M. B., Warner R., Murphy J. E. Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans). Environ Health Perspect. 1998 May;106(5):261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Skaare J. U., Tuveng J. M., Sande H. A. Organochlorine pesticides and polychlorinated biphenyls in maternal adipose tissue, blood, milk, and cord blood from mothers and their infants living in Norway. Arch Environ Contam Toxicol. 1988 Jan;17(1):55–63. doi: 10.1007/BF01055154. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol. 1994 May;141(2):295–299. doi: 10.1677/joe.0.1410295. [DOI] [PubMed] [Google Scholar]
- Williams J., Eckols K., Uphouse L. Estradiol and chlordecone interactions with the estradiol receptor. Toxicol Appl Pharmacol. 1989 May;98(3):413–421. doi: 10.1016/0041-008x(89)90170-1. [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Toniolo P. G., Lee E. W., Rivera M., Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993 Apr 21;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- Yeoh C. G., Schreck C. B., Fitzpatrick M. S., Feist G. W. In vivo steroid metabolism in embryonic and newly hatched steelhead trout (Oncorhynchus mykiss). Gen Comp Endocrinol. 1996 May;102(2):197–209. doi: 10.1006/gcen.1996.0061. [DOI] [PubMed] [Google Scholar]
- van den Hurk R., Slof G. A. A morphological and experimental study of gonadal sex differentiation in the rainbow trout, Salmo gairdneri. Cell Tissue Res. 1981;218(3):487–497. doi: 10.1007/BF00210109. [DOI] [PubMed] [Google Scholar]