Abstract
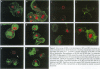
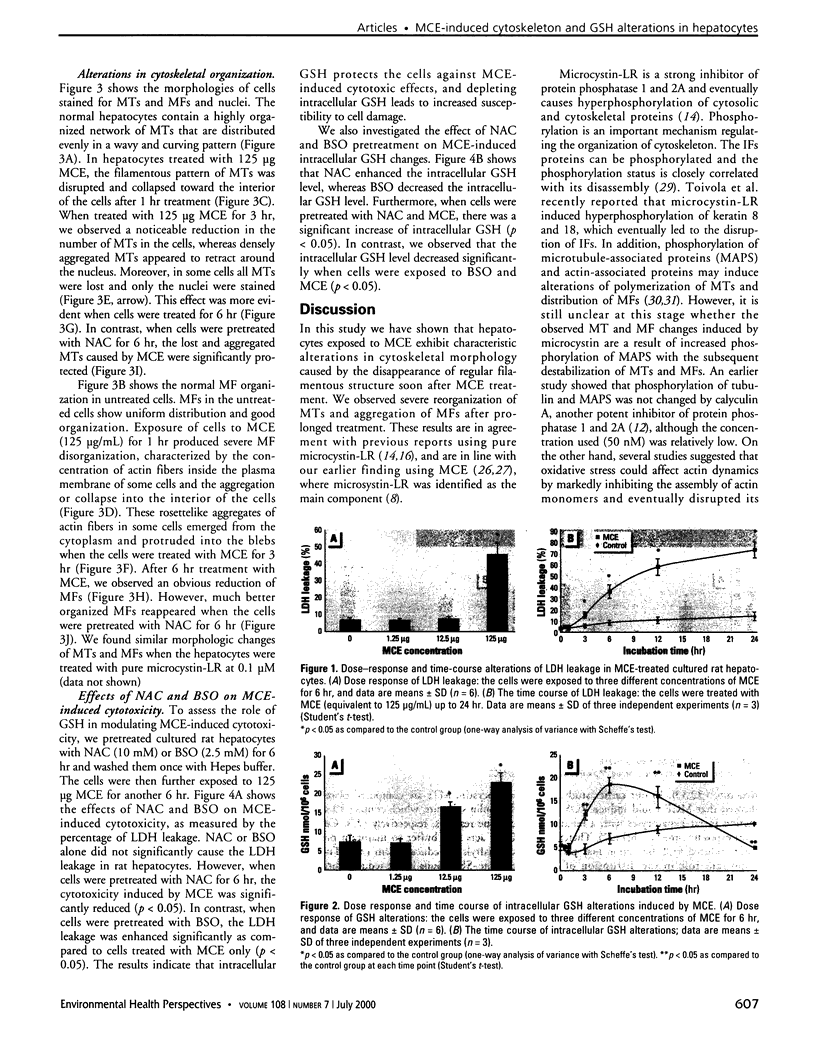
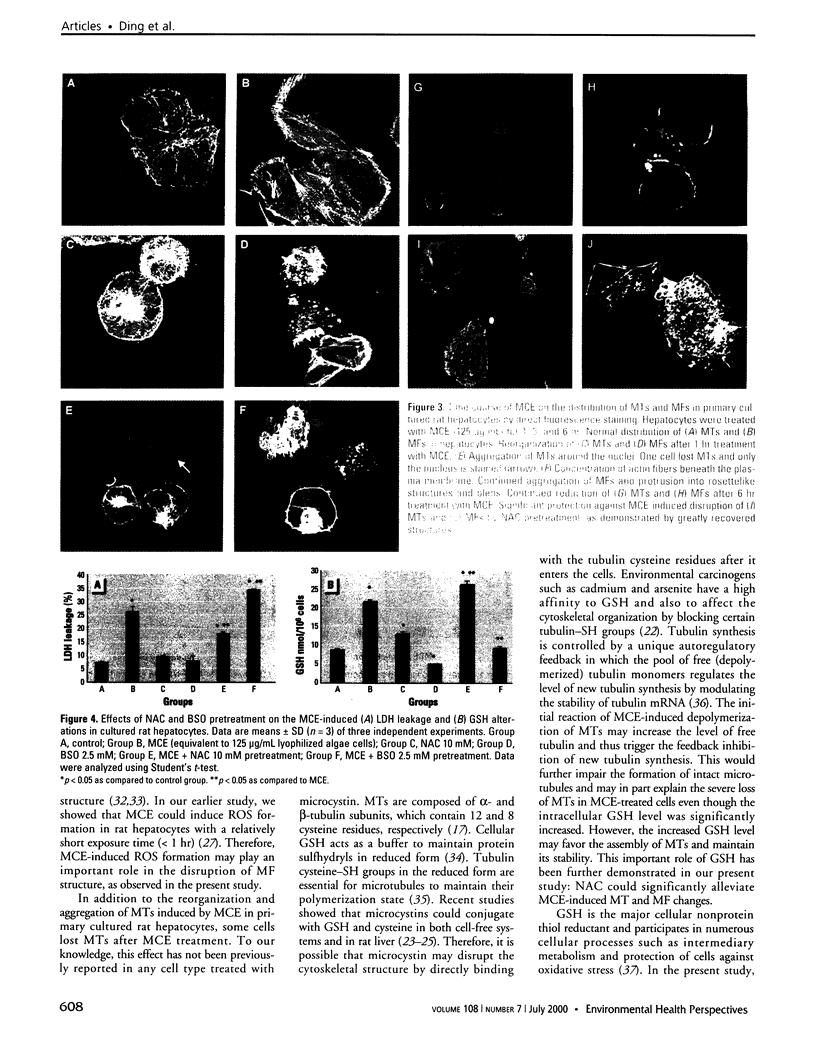
Microcystins are a group of highly liver-specific toxins, although their exact mechanisms of action remain unclear. We examined the effects of microcystic cyanobacteria extract (MCE) collected from a contaminated water source on the organization of cellular microtubules (MTs) and microfilaments (MFs) in hepatocytes. We also investigated the effects on lactate dehydrogenase (LDH) leakage and intracellular glutathione (GSH). Primary cultured rat hepatocytes exposed to MCE (equivalent to 125 microg/mL lyophilized algae cells) showed a characteristic disruption of MTs and MFs in a time-dependent manner. Under these conditions, MCE caused aggregation of MTs and MFs and a severe loss of MTs in some cells. Moreover, MCE-induced cytoskeletal alterations preceded the LDH leakage. On the other hand, the treatment of cells with MCE led to a dose-dependent increase of intracellular GSH. However, time-course study showed a biphasic change of intracellular GSH levels with a significant increase in the initial stage followed by a decrease after prolonged treatment. Furthermore, pretreatment with N-acetylcystein (NAC), a GSH precursor, significantly enhanced the intracellular GSH level and decreased the MCE-induced cytotoxicity as well as cytoskeleton changes. In contrast, buthionine-(S, R)-sulfoximine, a specific GSH synthesis inhibitor, increased the cell susceptibility to MCE-induced cytotoxicity by depleting the intracellular GSH level. These findings suggest that intracellular GSH plays an important role in MCE-induced cytotoxicity and cytoskeleton changes in primary cultured rat hepatocytes. Increasing intracellular GSH levels protect cells from MCE-induced cytotoxicity and cytoskeleton changes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Brugg B., Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991 Aug;114(4):735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael W. W. The toxins of cyanobacteria. Sci Am. 1994 Jan;270(1):78–86. doi: 10.1038/scientificamerican0194-78. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Ding W. X., Shen H. M., Shen Y., Zhu H. G., Ong C. N. Microcystic cyanobacteria causes mitochondrial membrane potential alteration and reactive oxygen species formation in primary cultured rat hepatocytes. Environ Health Perspect. 1998 Jul;106(7):409–413. doi: 10.1289/ehp.98106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W. X., Shen H. M., Zhu H. G., Lee B. L., Ong C. N. Genotoxicity of microcystic cyanobacteria extract of a water source in China. Mutat Res. 1999 Jun 25;442(2):69–77. doi: 10.1016/s1383-5718(99)00064-9. [DOI] [PubMed] [Google Scholar]
- Ding W. X., Shen H. M., Zhu H. G., Ong C. N. Studies on oxidative damage induced by cyanobacteria extract in primary cultured rat hepatocytes. Environ Res. 1998 Jul;78(1):12–18. doi: 10.1006/enrs.1998.3843. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Brautigan D. L., Vallee R., Olmsted J., Fujiki H., Goldman R. D. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J. E., Paatero G. I., Meriluoto J. A., Codd G. A., Kass G. E., Nicotera P., Orrenius S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp Cell Res. 1989 Nov;185(1):86–100. doi: 10.1016/0014-4827(89)90039-6. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Toivola D., Meriluoto J. A., Karaki H., Han Y. G., Hartshorne D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1347–1353. doi: 10.1016/s0006-291x(05)80936-2. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Honkanen R. E., Zwiller J., Moore R. E., Daily S. L., Khatra B. S., Dukelow M., Boynton A. L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem. 1990 Nov 15;265(32):19401–19404. [PubMed] [Google Scholar]
- Jochimsen E. M., Carmichael W. W., An J. S., Cardo D. M., Cookson S. T., Holmes C. E., Antunes M. B., de Melo Filho D. A., Lyra T. M., Barreto V. S. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998 Mar 26;338(13):873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- Kletsas D., Barbieri D., Stathakos D., Botti B., Bergamini S., Tomasi A., Monti D., Malorni W., Franceschi C. The highly reducing sugar 2-deoxy-D-ribose induces apoptosis in human fibroblasts by reduced glutathione depletion and cytoskeletal disruption. Biochem Biophys Res Commun. 1998 Feb 13;243(2):416–425. doi: 10.1006/bbrc.1997.7975. [DOI] [PubMed] [Google Scholar]
- Kondo F., Ikai Y., Oka H., Okumura M., Ishikawa N., Harada K., Matsuura K., Murata H., Suzuki M. Formation, characterization, and toxicity of the glutathione and cysteine conjugates of toxic heptapeptide microcystins. Chem Res Toxicol. 1992 Sep-Oct;5(5):591–596. doi: 10.1021/tx00029a002. [DOI] [PubMed] [Google Scholar]
- Kondo F., Matsumoto H., Yamada S., Ishikawa N., Ito E., Nagata S., Ueno Y., Suzuki M., Harada K. Detection and identification of metabolites of microcystins formed in vivo in mouse and rat livers. Chem Res Toxicol. 1996 Dec;9(8):1355–1359. doi: 10.1021/tx960085a. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Sakai H. Role of tubulin-SH groups in polymerization to microtubules. Functional-SH groups in tubulin for polymerization. J Biochem. 1974 Sep;76(3):651–654. doi: 10.1093/oxfordjournals.jbchem.a130609. [DOI] [PubMed] [Google Scholar]
- Leung M. F., Chou I. N. Relationship between 1-chloro-2,4-dinitrobenzene-induced cytoskeletal perturbations and cellular glutathione. Cell Biol Toxicol. 1989 Jan;5(1):51–66. doi: 10.1007/BF00141064. [DOI] [PubMed] [Google Scholar]
- Li W., Chou I. N. Effects of sodium arsenite on the cytoskeleton and cellular glutathione levels in cultured cells. Toxicol Appl Pharmacol. 1992 May;114(1):132–139. doi: 10.1016/0041-008x(92)90105-2. [DOI] [PubMed] [Google Scholar]
- Li W., Zhao Y., Chou I. N. Alterations in cytoskeletal protein sulfhydryls and cellular glutathione in cultured cells exposed to cadmium and nickel ions. Toxicology. 1993 Jan 29;77(1-2):65–79. doi: 10.1016/0300-483x(93)90138-i. [DOI] [PubMed] [Google Scholar]
- Meister A. New aspects of glutathione biochemistry and transport: selective alteration of glutathione metabolism. Fed Proc. 1984 Dec;43(15):3031–3042. [PubMed] [Google Scholar]
- Milzani A., Dalledonne I., Vailati G., Colombo R. Paraquat induces actin assembly in depolymerizing conditions. FASEB J. 1997 Mar;11(4):261–270. doi: 10.1096/fasebj.11.4.9068615. [DOI] [PubMed] [Google Scholar]
- Miura G. A., Robinson N. A., Geisbert T. W., Bostian K. A., White J. D., Pace J. G. Comparison of in vivo and in vitro toxic effects of microcystin-LR in fasted rats. Toxicon. 1989;27(11):1229–1240. doi: 10.1016/0041-0101(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Pace J. G., Robinson N. A., Miura G. A., Matson C. F., Geisbert T. W., White J. D. Toxicity and kinetics of [3H]microcystin-LR in isolated perfused rat livers. Toxicol Appl Pharmacol. 1991 Mar 1;107(3):391–401. doi: 10.1016/0041-008x(91)90303-v. [DOI] [PubMed] [Google Scholar]
- Pflugmacher S., Wiegand C., Oberemm A., Beattie K. A., Krause E., Codd G. A., Steinberg C. E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochim Biophys Acta. 1998 Nov 27;1425(3):527–533. doi: 10.1016/s0304-4165(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Pouria S., de Andrade A., Barbosa J., Cavalcanti R. L., Barreto V. T., Ward C. J., Preiser W., Poon G. K., Neild G. H., Codd G. A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet. 1998 Jul 4;352(9121):21–26. doi: 10.1016/s0140-6736(97)12285-1. [DOI] [PubMed] [Google Scholar]
- Rahilly M. A., Fleming S. A tumour promoter induces alterations in vinculin and actin distribution in human renal epithelium. J Pathol. 1992 Mar;166(3):283–288. doi: 10.1002/path.1711660311. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Bhattacharya R., Pant S. C., Bhaskar A. S. Toxicity evaluation of in vitro cultures of freshwater cyanobacterium Microcystis aeruginosa: I. Hepatotoxic and histopathological effects in rats. Biomed Environ Sci. 1995 Sep;8(3):254–264. [PubMed] [Google Scholar]
- Toivola D. M., Goldman R. D., Garrod D. R., Eriksson J. E. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J Cell Sci. 1997 Jan;110(Pt 1):23–33. doi: 10.1242/jcs.110.1.23. [DOI] [PubMed] [Google Scholar]
- Wickstrom M. L., Khan S. A., Haschek W. M., Wyman J. F., Eriksson J. E., Schaeffer D. J., Beasley V. R. Alterations in microtubules, intermediate filaments, and microfilaments induced by microcystin-LR in cultured cells. Toxicol Pathol. 1995 May-Jun;23(3):326–337. doi: 10.1177/019262339502300309. [DOI] [PubMed] [Google Scholar]
- Yu S. Z. Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepatol. 1995 Nov-Dec;10(6):674–682. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- van Gorp R. M., Broers J. L., Reutelingsperger C. P., Bronnenberg N. M., Hornstra G., van Dam-Mieras M. C., Heemskerk J. W. Peroxide-induced membrane blebbing in endothelial cells associated with glutathione oxidation but not apoptosis. Am J Physiol. 1999 Jul;277(1 Pt 1):C20–C28. doi: 10.1152/ajpcell.1999.277.1.C20. [DOI] [PubMed] [Google Scholar]