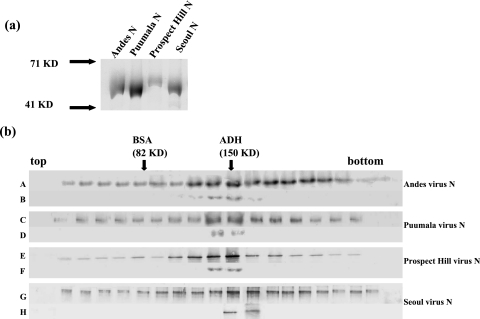
FIG. 1.
(a) N proteins from Andes, Puumala, Prospect Hill, and Seoul viruses were expressed in E. coli with C-terminal histidine fusion proteins. Each protein was purified by using Ni-NTA beads according to the manufacturer's protocol. Proteins were analyzed by SDS-polyacrylamide gel electrophoresis and visualized by Coomassie staining. (b) N proteins from Andes, Puumala, Prospect Hill, and Seoul viruses were sedimented through 10 to 45% sucrose gradients, and N was detected using Western blot analysis with anti-N antibody. Gradients A, C, E, and G correspond to N proteins from Andes, Puumala, Prospect Hill, and Seoul viruses, respectively. Gradients B, D, F, and H are trimeric N proteins that have been resedimented to examine the relative stability of trimeric N. Protein standards (bovine serum albumin [BSA] and alcohol dehydrogenase [ADH]) were run in parallel with the N samples, and arrows indicate the migration of those standards.