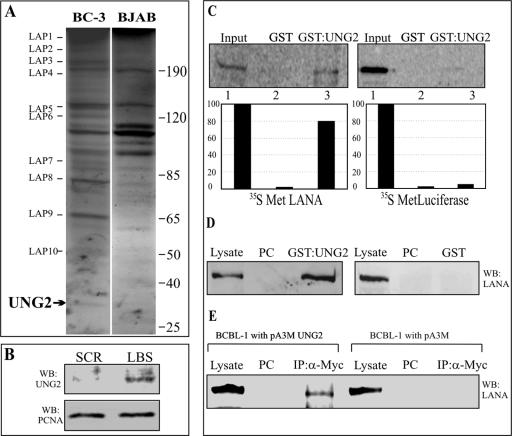
FIG. 1.
LANA bound to its cognate sequence in an affinity column interacts with host cell proteins. (A) Proteins eluted with buffer containing 500 mM NaCl from an LBS affinity column were resolved by 10% SDS-PAGE and stained with Coomassie brilliant blue. Bands specific to BC-3 cells (indicated as LAPs) were excised and sequenced. LAP12 was identified as UNG2. The values on the right are molecular sizes in kilodaltons. (B) UNG2 elutes from the affinity column containing LBS DNA but not from the SCR LBS DNA column. Equal amounts of BC-3 NE were incubated with either an LBS or an SCR LBS DNA affinity column, followed by thorough washing to remove any loosely bound protein. Protein eluted at 500 mM NaCl-containing buffer was Western blotted for detection of UNG2. PCNA shows that equal amounts of NE were present in the binding reaction mixture. (C) In vitro-translated LANA specifically binds to GST-UNG2. [35S]methionine-labeled LANA was incubated with either GST or GST-UNG2, and the bound fractions were quantified. The control protein (luciferase) did not bind to GST-UNG2. (D) Lysates from HEK293T cells expressing LANA-myc were incubated with either GST or GST-UNG2, and the bound fraction was analyzed by Western blotting with anti-myc antibody. LANA was precipitated with GST-UNG2 but not in the GST lane. PC, precleared with glutathione-Sepharose beads. (E) LANA coimmunoprecipitated with UNG2 from BCBL-1 cells. The pA3MUNG2 or pA3M vector only was transfected into BCBL-1 cells and immunoprecipitated (IP) with myc ascites. Western immunoblot assay detection with anti-LANA antibody showed coimmunoprecipitation of LANA from pA3MUNG2-transfected cells but not from cells transfected with the vector only. PC, precleared with protein A and protein G beads.