Abstract
Occult hepatitis C virus (HCV) infection is a type of recently identified chronic infection that is evidenced only by detection of HCV RNA in liver; patients consistently test negative for antibodies to HCV and HCV RNA in serum. Using ex vivo and in vitro measures of T-cell responses, we have identified functional virus-specific memory CD4+ and CD8+ T cells in the peripheral blood of patients with occult HCV infection. The features of the virus-specific T cells were consistent with immune surveillance functions, supporting previous exposure to HCV. In addition, the magnitudes of CD4+ and CD8+ T-cell responses were in parallel and correlated inversely with the extent of liver HCV infection. The detection of HCV-specific T cells in individuals in whom HCV RNA can persist in the liver despite the absence of viremia and antibodies indicates that HCV replication is prolonged in the face of virus-specific CD4+ and CD8+ T-cell responses. These findings demonstrate that HCV-specific cellular immune responses are markers not only of previous exposure to and recovery from HCV but also of ongoing occult HCV infection.
Current criteria for hepatitis C virus (HCV) infection include detection of specific antibodies by enzyme immunoassay and confirmation by immunoblot assay (40). Chronic HCV infection is established in approximately 50 to 80% of virus-exposed individuals, with 170 million people being infected worldwide (30). Patients in whom HCV persists usually remain positive for anti-HCV and HCV RNA in serum unless HCV is cleared from the circulation either spontaneously or under antiviral treatment (30, 41). Another type of chronic infection, occult HCV, has recently been identified in a group of patients who have abnormal liver function tests and histological damage (2). Occult HCV infection is characterized by the presence of HCV RNA in the liver in patients who consistently test negative for antibodies to HCV and HCV RNA in serum. Compared with chronic hepatitis C, occult HCV infection seems to be a less aggressive form of the disease caused by the hepatitis C virus (25). A recent report suggests that interferon-based therapy may have potential benefits in the treatment of patients with occult HCV infection (24).
Virus-specific T-cell responses have been detected in the blood of HCV-seronegative healthy persons frequently exposed to HCV (16, 36) and in patients lacking humoral responses who have presumably recovered from acute hepatitis C (41). HCV-specific cellular responses can be detected in patients receiving antiviral therapy in association with HCV RNA clearance from the blood (5, 11, 12). In spite of this, detectable HCV may persist in the liver in so-called sustained responders to treatment (27, 29). Therefore, we questioned whether HCV-specific T-cell responses are detectable in patients with occult HCV infection.
(This work was presented in part at the 55th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, Mass., 29 October to 2 November 2004 [28a].)
MATERIALS AND METHODS
Study subjects.
Fifty patients with occult HCV infection were enrolled in this study. The selection criteria were having sustained abnormal liver function tests of unknown etiology for a minimum time of 12 months (tested every 3 months) prior to undergoing a liver biopsy. Thus, no etiology could be identified after exclusion of all known causes of liver disease on the basis of clinical, epidemiological, and laboratory data (HCV infection [anti-HCV and serum HCV RNA negative, as tested at least on two occasions] [2]), HBV infection [HBV surface antigen and serum HBV DNA negative], autoimmunity, metabolic and genetic disorders, alcohol intake, drug toxicity, etc.); all cases were negative for anti-human immunodeficiency virus antibodies. None of the patients had a clinical or biochemical history of acute hepatitis. There were no known risk factors for HCV infection. Thus, as reported previously (2), occult HCV infection was identified following detection of HCV RNA in liver tissue in patients who lacked serum HCV RNA (Amplicor HCV version 2.0; Roche Diagnostics, Branchburg, NJ; sensitivity of 50 IU/ml and specificity of 99%) and anti-HCV antibodies (INNOTEST-HCV Ab IV; Innogenetics, Ghent, Belgium) and who presented with abnormal liver function tests of unknown etiology. HCV RNA amplified from liver biopsies was genotyped by a standard methodology (INNO-LIPA HCV II; Innogenetics); all patients with occult HCV infection showed HCV1b (2).
A total of 141 patients with chronic hepatitis C (serum anti-HCV and HCV RNA positive) and 21 patients with cryptogenic liver disease (serum anti-HCV and HCV RNA negative and liver HCV RNA negative but with abnormal transaminase values) were also enrolled. Table 1 shows the characteristics of the patients. The clinical, laboratory, and histological features of patients with occult HCV infection versus patients with chronic hepatitis C are described in more detail elsewhere (25). The study was approved by the ethics committee of the institution and was conducted according to the Declaration of Helsinki on human experimentation. Informed consent was obtained from the patients.
TABLE 1.
Characteristics of the patients
| Parameter (unit) | Valuea for patients with:
|
P value | ||
|---|---|---|---|---|
| Occult HCV (n = 50) | Chronic hepatitis C (n = 141) | Cryptogenic liver disease (n = 21) | ||
| Age (yr) | 46.4 (43.0-49.8) | 47.0 (44.9-49.0) | 45.3 (32.7-47.9) | 0.33 |
| Gender (no. male/no. female) | 39/11 | 90/51 | 15/6 | 0.17 |
| Duration of disease (yr)b | 7.2 (3.6-10.8) | 7.4 (5.9-8.9) | 5.3 (4.1-6.5) | 0.070 |
| Alanine aminotransferase (IU/liter) | 70 (49-92) | 109 (93-125) | 69 (58-79) | <0.001 |
| Aspartate transaminase (IU/liter) | 40 (35-46) | 72 (61-83) | 45 (35-48) | <0.001 |
| Gamma-glutamyl transpeptidase (IU/liter) | 106 (81-131) | 67 (49-74) | 84 (70-99) | 0.089 |
| No. (%) with: | ||||
| Necroinflammation | 18 (36) | 141 (100) | 5 (24) | <0.001 |
| Fibrosis | 10 (20) | 110 (78) | 6 (29) | <0.001 |
| Cirrhosis | 2 (4) | 12 (9) | 2 (10) | 0.55 |
| Steatosis | 10 (20) | 14 (10) | 11 (52) | 0.065 |
Except as indicated, values are means and 95% confidence intervals.
Estimated duration of abnormal liver function tests since first alteration was detected.
Serum and peripheral blood mononuclear cells (PBMC) samples were collected on the same day of the liver biopsy and every 3 to 6 months thereafter when possible. The liver fragment was cut into two portions; one portion was used for histological diagnosis (35), and the other was embedded in RNAlater (Ambion Inc., Austin, TX) (within 30 seconds after obtaining the liver sample) and stored at −20°C until use. The presence of HCV RNA in liver tissue was tested by reverse transcription-PCR (RT-PCR) as described previously in detail (2). Briefly, 0.5 μg total RNA isolated from liver was reverse transcribed and amplified using the Access RT-PCR system (Promega, Madison, WI) with primers for the 5′ noncoding region of the HCV genome. The RT-PCR was carried out at 48°C for 45 min, followed by 2 min at 94°C and by the first amplification round, consisting of 30 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 68°C for 1 min. The second PCR was done with 2 μl of the product of the first PCR under the same conditions as for the first PCR. The sensitivity of this RT-PCR was 10 IU/ml (equivalent to nine copies/ml according to the conversion factor reported in reference 26). The presence of HCV RNA positive strand in liver biopsies was confirmed by in situ hybridization in paraffin-embedded liver sections (4 μm) (2) with a cRNA probe obtained by in vitro transcription of the pC5′NCR plasmid, which contains the complete 5′ noncoding region of the HCV genome (34).
T-cell proliferation assay.
PBMCs isolated from heparinized blood by gradient centrifugation were suspended in RPMI 1640 medium (Imperial Labs, Andover, United Kingdom) with supplements and cultured in triplicate (1.0 × 105/100 μl) with or without 1 μg/ml HCV proteins (core, NS3/helicase, NS4, and NS5A/NS5B; Mikrogen GmbH, Munich, Germany) or Staphylococcus aureus enterotoxin B (10 μg/ml; Sigma Chemical Co., St. Louis, MO) as a positive control. Cell proliferation was quantitated by [3H]thymidine uptake assay as described previously (32).
CD4+ T-cell lines.
HCV-specific CD4+ T-cell lines were generated as described elsewhere (6) by a single round of stimulation (5 × 106 PBMCs) with recombinant NS3 and NS4 proteins (1 μg/ml) in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum and 50 U/ml of recombinant interleukin-2 (IL-2) (Sigma). After 10 to 14 days of culture, T-cell lines were assayed for specificity by means of a proliferation assay.
In addition, T cells (1.0 to 2.0 × 106) plus 0.5 to 1.0 × 105 mitomycin C (25 μg/ml)-treated autologous PBMCs as feeder cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum for 16 h at 37°C, 5% CO2, and humidity, either without stimulation (spontaneous secretion for background responses), with 10 μg/ml of HCV NS3 and NS4 proteins (Mikrogen), with 10 μg/ml NS3(1248-1260) peptide, with 10 μg/ml of human cytomegalovirus (CMV) antigen lysate (Biodesign, Saco, ME), or with 10 μg/ml of S. aureus enterotoxin B as a positive control. Gamma interferon (IFN-γ)-producing CD4+ T cells were detected by flow cytometry using cytokine secretion assays as described previously (32), following the supplier's instructions (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Samples were counterstained with combinations of anti-CD3-phycoerythrin-cyanin 5 (PC5), anti-CD4-PC5/anti-CD4-phycoerythrin-Texas Red X (ECD), anti-CD14-PC5/anti-CD14-ECD, anti-CD45RO-ECD, anti-CD62L-fluorescein isothiocyanate (FITC), and anti-CD69-PC5, plus the viability stain 7-amino-actinomycin D (all from Beckman-Coulter). Cytokine-producing CD4+ T cells were then enumerated by flow cytometry among CD4+ T cells gated for living lymphocytes (at least 30,000 to 50,000 CD4+ events were acquired) after subtracting the frequency of spontaneous cytokine-secreting cells in unstimulated cultures. Isotype controls were used to establish the vertical and horizontal boundaries that define regions of positive versus negative events.
Detection of Th1/Th2 cytokines.
A fluorescent bead immunoassay was used for quantitative detection by flow cytometry of IFN-γ, IL-5, and IL-10 (human Th1/Th2 cytokine FlowCytomix; Bender MedSystems, Vienna, Austria) in culture supernatants. PBMC-derived T-cell lines were restimulated for 16, 48, or 72 h with 1 μg/ml HCV proteins. Supernatants were collected, centrifuged, and stored at −20°C until assay to avoid loss of bioactivity. Assay sensitivities are as follows: IFN-γ, 10.5 pg/ml; IL-5, 7.9 pg/ml; and IL-10, 10.3 pg/ml.
Typing of HLA class I A2 and HLA class II Dw4 molecules.
PBMCs were stained with the FITC-conjugated monoclonal antibodies BB7.2 (BD PharMingen, San Diego, CA) and NFLD.D11 (TNB Labs., St. John's, Newfoundland, Canada), which recognize HLA-A2 and HLA-DR4.Dw4 (HLA-DRB1*0401/0413) antigens, respectively, and analyzed by flow cytometry on an Epics XL cytometer using Expo32-ADC software (Beckman-Coulter, San Diego, CA).
Enumeration and phenotype of DR4/HCV-positive CD4+ T cells.
PBMC-derived or antigen-expanded T lymphocytes (at least 1.0 × 106) were stained for 2 h at 37°C, 5% CO2, and humidity with the DR4/HCV NS3(1248-1260) tetramer (amino acid sequence, GYKVLVLNPSVAA) (7, 8) conjugated with phycoerythrin (PE) (Beckman-Coulter, Fullerton, CA). Cells were stimulated overnight with the specific peptide (10 μg/ml); brefeldin A was added for the last 4 h of incubation. Subsequently cells were stained with anti-CD4-ECD and anti-CD69-PC5, fixed, permeabilized, and then incubated for 30 min at room temperature, protected from light, with anti-IFN-γ-FITC (iTAg MHC Tetramer IFN-γ kit; Beckman-Coulter), anti-tumor necrosis factor alpha (anti-TNF-α) (Miltenyi), or anti-Ki67-FITC (Dako A/S, Glostrup, Denmark); finally, cells were stored for 1 h at 4°C prior to analysis by flow cytometry. Frequencies of tetramer-positive cells were determined among CD4+ T cells gated for living lymphocytes (at least 30,000 to 50,000 CD4+ events were acquired). Isotype controls were used to establish the vertical and horizontal boundaries that define regions of positive versus negative events.
Enumeration and phenotype of A2/HCV-positive CD8+ T cells.
PBMCs (1.0 × 106) from patients were stained for 40 min on ice in the dark with HLA-A2/HCV peptide multimers conjugated with PE [NS3(1073-1081) (amino acid sequence, CVNGVCWTV) or NS3(1406-1414) (amino acid sequence, KLVALGINAV] (Proimmune, Oxford, United Kingdom). Cells were then stained with anti-CD8-ECD, anti-CD38-FITC, anti-CD14-PC5, and the viability dye 7-amino-actinomycin D and analyzed immediately by flow cytometry for enumeration of HCV-positive CD8+ T cells gated for living lymphocytes. In addition, 1.0 × 106 cells were labeled for 30 min at room temperature, protected from light, with the mixture of HLA-A2/HCV multimers NS3(1073-1081) and NS3(1406-1414) conjugated with PE, followed by stimulation with specific peptides (5 μg/ml of each) for 1 h at 37°C, 5% CO2, and humidity; brefeldin A was then added and the incubation continued for another 4 h. Subsequently cells were stained with anti-CD8-ECD and anti-CD69-PC5, fixed, permeabilized, and then incubated for 30 min at room temperature protected from light with anti-IFN-γ-FITC (iTAg MHC Tetramer IFN-γ kit; Beckman-Coulter) or with anti-TNF-α (Miltenyi). Cells were stored at 4°C, protected from light, for a minimum of 1 h prior to analysis by flow cytometry. Frequencies of cytokine-positive cells were determined among CD8+ T cells gated for living lymphocytes (at least 30,000 to 50,000 CD8+ events were acquired). A no-HCV-peptide (medium) or influenza A virus matrix peptide (sequence, GILGFVFTL) was employed during antigen stimulation to control for nonspecific binding of cytokines.
Statistical analysis.
Results were analyzed by nonparametric tests using the SPSS program (version 9.0; SPSS Inc., Chicago, IL). The chi-square test (or Fisher's exact test when applicable) was used to compare frequencies. Correlations were done using the Spearman's rank correlation coefficient. All P values reported are two tailed.
RESULTS
HCV-specific CD4+ T-cell responses are detected in the peripheral blood in patients with occult HCV infection.
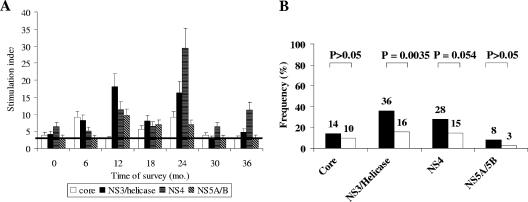
First, we measured in vitro CD4+ T-cell proliferative responses prospectively at 3- to 6-month intervals and identified virus-specific responses in patients with occult HCV infection. On repeated serial testing within an initial 1-year survey, some fluctuations were observed in CD4+ T-cell proliferative responses compared with the baseline samples (from positive to negative and vice versa). An extended follow-up (range, 6 to 24 months) confirmed such fluctuations in CD4+ T-cell proliferative responses in patients with occult HCV infection (Fig. 1A), although the differences at each time point were not statistically significant. Thus, overall, 26 out of 50 patients (52%) had HCV-specific CD4+ T-cell responses. Among these 26 CD4+ T-cell responders, proliferative responses were maintained as positive in nine occult HCV-infected patients throughout the entire follow-up, but they presented as alternating positive or negative episodes in 17 patients; 24 occult HCV-infected patients tested repeatedly negative and were considered CD4+ T-cell nonresponders.
FIG. 1.
HCV-specific CD4+ T-cell proliferative response in peripheral blood mononuclear cells. (A) Time course of proliferative responses in 26 CD4+ T-cell responder patients with occult HCV infection, expressed as stimulation indices (SI). Bars represent mean SI of T-cell responses to the HCV protein with standard errors of the means. The horizontal line represents the cutoff for a positive response (SI of >3). (B) Patients with occult HCV infection (black bars) (n = 50) were compared with chronic hepatitis C patients (white bars) (n = 141) for frequencies of virus-specific CD4+ T-cell proliferation in response to recombinant HCV core, NS3/helicase, NS4, and NS5A/NS5B proteins.
HCV-specific CD4+ T-cell proliferative responses were significantly more frequent in patients with occult HCV infection than in the group with chronic hepatitis C (37/141, 26.2%; P = 0.0016 by chi-square test) or in individuals with cryptogenic liver disease (1/21, 4.8%; P < 0.001 by chi-square test). Fluctuations in CD4+ T-cell proliferative responses were also noted in patients with chronic hepatitis C (data not shown). HCV-specific T cells from patients with occult HCV infection proliferated more commonly in response to NS3 and NS4 proteins (Fig. 1B). The only CD4+ T-cell responder patient with cryptogenic liver disease had a proliferative response against core protein alone (data not shown).
CD4+ T cells are capable of mediating effector functions.
To examine the features of the virus-specific T cells found in patients with occult HCV infection, T cells from 23 out of the 26 CD4+ T-cell responders were expanded in vitro by short-term culturing in the presence of NS3 and NS4 proteins and IL-2. It must be noted that this technical approach did not induce primary HCV-specific CD4+ T-cell responses in any of the uninfected individuals tested (data not shown) (6). Instead, this procedure allowed expansion of preexisting memory CD4+ T cells, although the phenotype of the specific cells might have changed in culture during antigen-induced activation (17). Thus, HCV-specific T-cell lines were obtained in 12/23 patients (52%), of whom T cells were expanded in 8 (67%), 1 (8%), and 3 (25%) in response to NS3 alone, NS4 alone, and NS3 and NS4, respectively.
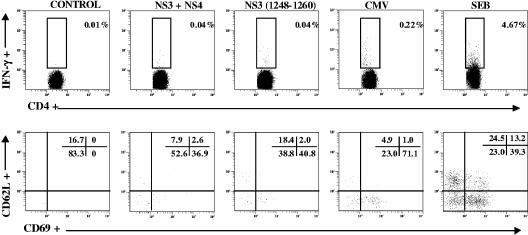
Next, PBMC-derived T-cell lines from these 12 patients were restimulated with specific protein or peptide and analyzed for IFN-γ production by flow cytometry-based secretion assay. Discrete numbers of HCV-specific IFN-γ-secreting CD4+ T cells were visualized in these 12 occult HCV-infected patients (median of 0.025%; range, 0.01% to 0.17%) after subtracting background responses in unstimulated cells. Thus, very low percentages of IFN-γ responses were seen even after antigen expansion. As shown in Fig. 2, IFN-γ responses to HCV restimulation were 0.03% above background in control CD4+ T cells expanded from the peripheral blood. Analysis of the homing receptor CD62L showed that most IFN-γ-secreting CD4+ T cells lacked CD62L expression (Fig. 2, lower panels), although IFN-γ responses were also found in the CD62L+ fraction of NS3-specific CD4+ T cells. It must be noted that during the antigen stimulation period, memory CD4+ T cells may have down-regulated CD62L (4, 33). In five CMV-seropositive occult HCV-infected individuals, average numbers of CMV-specific IFN-γ-secreting CD4+ T cells ranged from 0.04% to 0.22% (that is, 0.21% above background in Fig. 2). Thus, IFN-γ expression may reflect a recent encounter with cognate antigen in vivo as a result of ongoing HCV exposure. In contrast to patients with occult HCV infection, HCV-specific CD4+ T-cell lines could not be established in most of the chronic hepatitis C patients analyzed or in uninfected individuals with cryptogenic liver disease, and no functional response was detected.
FIG. 2.
Identification of HCV-specific cytokine-secreting CD4+ T cells in T-cell lines generated by in vitro stimulation with HCV NS3 and NS4 proteins. Flow cytometric analysis show the presence of virus-specific CD4+ T cells that secrete IFN-γ (upper panels). Gating for virus-specific IFN-γ-secreting CD4+ T cells (lower panels) indicates expression of the recent activation marker CD69 upon restimulation with HCV proteins or with an NS3-derived peptide containing a CD4+ T-cell epitope, compared with unstimulated (medium-treated) T cells; most virus-specific IFN-γ-secreting CD4+ T cells lack CD62L expression. The results shown are representative of experiments conducted with T-cell lines from 12 CD4+ T-cell responder patients. Numbers in the upper right corners refer to the percentages of marker-positive cells in the region or quadrant.
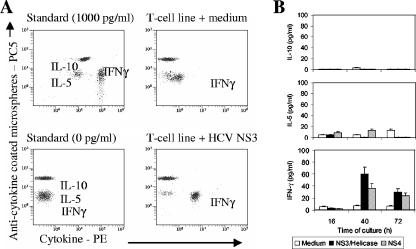
To further profile the functionality of HCV-specific CD4+ T-cell lines, the cytokine production pattern was analyzed by flow cytometry-based assay measuring the levels of IFN-γ, IL-5, and IL-10 in the supernatants of cultured T cells in response to HCV proteins. In all PBMC-derived T-cell lines from occult HCV-infected patients analyzed, only IFN-γ production was increased above the level of spontaneous production (Fig. 3A). This was equally true at the different time points assayed after restimulation (Fig. 3B), indicating that HCV-specific responder CD4+ T-cell lines show a type 1 helper T-cell pattern of cytokine production (Fig. 2).
FIG. 3.
Th1/Th2 cytokine profiling of HCV-specific CD4+ T-cell lines. Cytokines in supernatants of cultured T-cell lines restimulated with HCV NS3 or NS4 protein were detected and quantitated by flow cytometry. (A) IFN-γ production is up-regulated by NS3 compared with the control (medium). (B) IFN-γ, but not IL-5 or IL-10, secretion increases upon NS3 or NS4 restimulation compared with medium alone at any of the three culture time points assayed, confirming a Th1 pattern of responder CD4+ T-cell lines. Histograms represent the means ± standard errors of the means from experiments conducted with three patients.
HLA class II tetramer detection and phenotypic analysis of virus-specific CD4+ T cells.
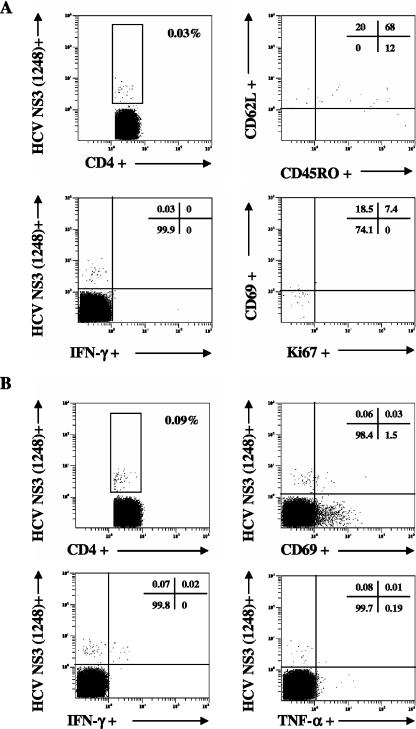
The presence of virus-specific CD4+ T cells was further analyzed by HLA class II tetramer staining of PBMC-derived T cells from 10 HLA-DR4.Dw4-positive patients. Direct enumeration revealed discrete frequencies of DR4-HCV NS3(1248) tetramer-positive CD4+ T cells in six patients with occult HCV infection (median of 0.09%; range, 0.02% to 0.30%), whereas tetramer-positive CD4+ T cells were undetectable in another four individuals assessed. As shown in Fig. 4, ex vivo HCV tetramer-positive CD4+ T cells showed coexpression of CD45RO and CD62L, a phenotype consistent with central memory CD4+ T cells (10). In addition, most DR4-HCV NS3(1248)-specific CD4+ T cells had no or limited recycling capacity (Ki67−/+dim); only a subset became activated and secreted cytokines (IFN-γ and/or TNF-α) upon restimulation with the cognate peptide (Fig. 4), consistent with immune surveillance functions. In contrast to patients with occult HCV infection, no HLA class II tetramer staining of HLA-matched, HCV-uninfected control PBMCs (from three patients with cryptogenic liver disease) was observed with the DR4-HCV NS3(1248) tetramer. In addition, HCV tetramer-positive CD4+ T cells were undetectable in PBMCs from any of the chronic hepatitis C patients analyzed (data not shown).
FIG. 4.
Fine specificity of CD4+ T cells. DR4-HCV NS3-positive CD4+ T cells were enumerated by flow cytometry through labeling with HLA class II tetramers and costaining for surface or intracellular molecules. CD4+ T cells were analyzed for expression of the homing receptor CD62L and the memory marker CD45RO, activation and proliferation markers (CD69 and Ki67, respectively), and production of effector cytokines (IFN-γ and TNF-α). (A) Ex vivo analysis of PBMC-derived CD4+ T cells. Functional and phenotypic characterization of DR4-HCV NS3-positive CD4+ T cells reflects their in vivo condition. (B) Detection and functional analysis of DR4-HCV NS3-positive CD4+ T cells following expansion by culture in the presence of HCV antigens and IL-2. The results are representative of experiments conducted with six HCV NS3-specific CD4+ T-cell responder patients. Numbers in the upper right corners refer to the percentage of positive cells in each quadrant.
HCV-specific CD8+ T cells are present in the peripheral blood in patients with occult HCV infection.
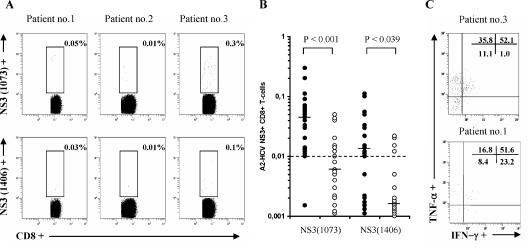
The HCV-specific CD8+ T-cell response was assessed ex vivo by HLA class I multimer staining of blood-derived PBMCs in the subset of HLA-A2-positive patients (n = 20). Flow cytometric analysis allowed detection of HCV NS3-specific CD8+ T cells at frequencies equal to or greater than 0.01% after subtraction of background responses (Fig. 5A). All the HLA-A2-positive patients with occult HCV infection analyzed showed discrete amounts of HCV-specific CD8+ T cells to at least one of the two HLA class I A2-HCV tetramers investigated: NS3(1073) specific, 95%; NS3(1406) specific, 60% (Fig. 5B). In contrast, a minority of chronic hepatitis C patients showed HCV-specific CD8+ T cells [NS3(1073) specific, 40%, (P < 0.001 by Fisher's exact test versus patients with occult HCV infection); NS3(1406) specific, 20% (P = 0.01 by Fisher's exact test versus patients with occult HCV infection)]. When detectable, HCV-specific CD8+ T cells were present at significantly lower frequencies than in occult HCV-infected patients [NS3(1073) specific, P < 0.001; NS3(1406) specific, P = 0.039]. On the other hand, the functional analysis indicated that HCV-specific CD8+ T cells produced the effector cytokines IFN-γ and/or TNF-α upon HCV NS3 peptide restimulation (Fig. 5C).
FIG. 5.
Analysis of HCV-specific CD8+ T cells in patients with occult HCV and chronic hepatitis C. (A) Visualization of HCV-specific CD8+ T cells in HLA-A2-positive patients with occult HCV infection through labeling with A2-HCV NS3(1073-1081) (upper panels) or NS3(1406-1415) (lower panels) multimers in three representative patients. Numbers refer to the percentage of marker-positive cells. (B) Comparison of frequencies (%) of A2-HCV NS3-positive CD8+ T cells in patients with occult HCV infection (black circles) (n = 20) and those with chronic hepatitis C (white circles) (n = 20). Horizontal bars indicate the median values; the dashed line represents the threshold for positive versus negative results. (C) HCV-specific CD8+ T cells produce effector cytokines IFN-γ and/or TNF-α. Results shown are from patients 3 and 1; numbers in the upper right corners refer to the percentage of positive cells in each quadrant.
Virus-specific cellular immune responses and HCV persistence in liver.
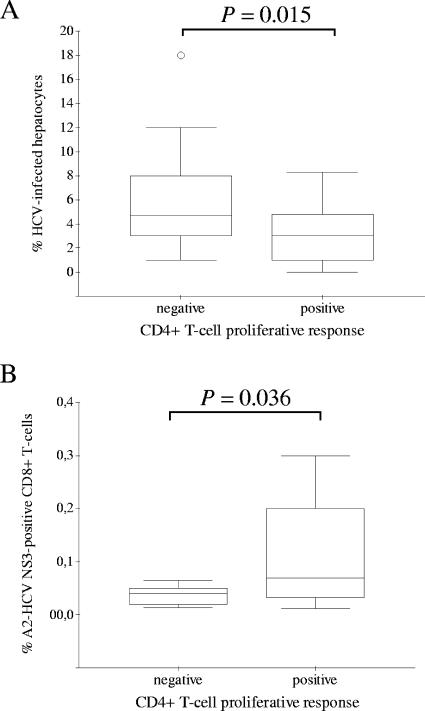
Patients with occult HCV infection were categorized as CD4+ T-cell responders (those who ever had had a positive CD4+ T-cell proliferative response) (n = 26) and CD4+ T-cell nonresponders (those who never mounted a detectable CD4+ T-cell proliferative response) (n = 24). Interestingly, the percentage of infected hepatocytes (that is, positive for genomic HCV RNA by in situ hybridization) was significantly lower (P = 0.015) in CD4+ T-cell responder patients than in CD4+ T-cell nonresponders (Fig. 6A). Also, significantly higher counts of HCV-specific CD8+ T cells were found in CD4+ T-cell responder patients (Fig. 6B). In addition, a statistically significant inverse correlation was observed between counts of HCV-specific CD8+ T cells and the percentage of infected hepatocytes (rs = −0.572; P = 0.034).
FIG. 6.
Relationship between hepatic HCV infection and cellular immune responses. Box plot representations of the percentages of HCV-infected hepatocytes (A) or circulating HCV NS3-specific CD8+ T cells (B) in occult HCV-infected patients with negative and positive CD4+ T-cell proliferation in peripheral blood are shown. Outliers are represented by single circles.
On the other hand measures of liver histology (average scores of necroinflammation and fibrosis or steatosis) tended to be greater, although not significantly, in CD4+ or CD8+ T-cell responder patients with occult HCV infection (data not shown).
DISCUSSION
In this study we demonstrated that patients with occult HCV infection have HCV-specific cellular immune responses in peripheral blood. Thus, functional virus-specific memory CD4+ and CD8+ T cells are present in the peripheral blood of individuals in whom detectable HCV RNA can persist in the liver despite the absence of viremia and antibodies. In addition, the features of the virus-specific T cells are consistent with immune surveillance functions (10, 17), supporting previous exposure to HCV. Furthermore, HCV-specific T-cell responses are significantly more frequent in occult HCV infection than in chronic hepatitis C.
Chronic infection with HCV is generally associated with weak and sometimes transient T-cell responses, whereas viremia clearance is accompanied by up-regulation of virus-specific cellular responses (19, 20, 31, 42, 43). Not all patients with occult HCV infection displayed specific CD4+ T-cell responses. One possible explanation is the fact that all our patients with occult HCV infection had genotype 1b (2), which seems to induce T-cell responses less frequently than genotype 2 or 3 (1, 11, 14). The present study does not exclude the presence of additional T-cell reactivities not yet investigated, and thus a detailed comprehensive analysis is warranted. Alternatively, the very low frequencies of HCV-specific CD4+ T cells detected even after antigen expansion might indicate that these are “rare” virus-specific cells, as reported recently (37). In occult HCV infection, detectable HCV persists in the liver despite the absence of viremia (2), which may represent a seronegative virus carrier state, and thus only partial HCV-specific T-cell responses may have been mounted. Cellular responses have been reported in seronegative individuals (for example, in sexual contacts of patients with acute hepatitis C) (13).
HCV tetramer-positive CD4+ T cells were undetectable in PBMCs from patients with chronic hepatitis C, in contrast to the case for occult HCV-infected patients. These results confirm that such T cells are either absent in chronic hepatitis C or present at frequencies below the threshold of the assays used to detect virus-specific cellular responses, as has been recently reported (7). It will be worth trying ultrasensitive methods for detection and phenotyping of rare HCV-specific CD4+ T cells, as recently described (37). Also, HCV NS3-specicific CD8+ T cells were detectable in fewer chronic hepatitis C patients (40%) (22) and at reduced frequencies compared with occult HCV infection. On the other hand, the magnitudes of CD4+ and CD8+ T-cell responses were in parallel and correlated inversely with the extent of liver HCV infection in patients with occult HCV (9). Therefore, the finding of HCV-specific T cells in occult HCV-infected patients suggests that virus persistence in the liver in aviremic individuals can cause a cellular response which may be maintained by HCV replication within infected hepatocytes (or in circulating immune cells as well), producing discrete amounts of antigens (2, 3, 21, 27).
The lack of anti-HCV antibodies and HCV RNA in the sera of patients with occult HCV infection differentiates this state from HCV-seropositive chronic infection. Given the latent nature of the disease prior to clinical presentation, the actual rate of HCV transmission and persistence may be underscored. Hepatitis C virus remains a large health care problem worldwide (18). However, in the absence of known risk factors for infection, it may be difficult to understand the epidemiology of occult HCV and cryptogenic liver disease (15). Important implications include the need to trace the origin and spread of the infection and to determine whether it is community acquired and if a common mode of HCV acquisition is being a household contact of an occult HCV-infected patient. Thus, epidemiological studies to assess viral transmission are ongoing.
Together, the findings of the present work demonstrate that patients with occult HCV infection have an HCV-specific cellular immune response in the peripheral blood, supporting the presence of HCV infection despite the absence of detectable serum HCV RNA and antibodies. Patients with occult HCV are similar to those who have previously been considered to have recovered because they consistently test HCV RNA negative without detectable HCV-specific humoral responses (41). The presence of HCV-specific T-cell responses could be important to keep HCV in the infected liver under control (39). This would explain the absence of HCV RNA in serum and provide at least partial protective immunity (23). Nevertheless, because detectable HCV persists in the liver, the role of these cellular responses in virus elimination may need reevaluation.
Our results and those of others (13, 28, 38) indicate that it may be important to introduce sensitive assays for virus-specific cellular immune responses to test for HCV exposure in diverse populations, such HCV-seronegative individuals with liver disease, hemodialysis patients, or blood donors. These analyses could provide important information about the status of HCV, with potential implications in the clinical setting and in the possible modification of our understanding of the natural history of HCV infection.
Acknowledgments
The authors have no conflicting financial interests.
This work has been supported in part by a grant from “Fundación de Investigaciones Biomédicas,” Madrid, Spain.
REFERENCES
- 1.Candotti, D., J. Temple, F. Sarkodie, and J. P. Allain. 2003. Frequent recovery and broad genotype 2 diversity characterize hepatitis C virus infection in Ghana, West Africa. J. Virol. 77:7914-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo, I., M. Pardo, J. Bartolomé, N. Ortiz-Movilla, E. Rodríguez-Iñigo, S. de Lucas, C. Salas, J. A. Jiménez-Hefferman, A. Pérez-Mota, J. Graus, J. M. López-Alcorocho, and V. Carreño. 2004. Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J. Infect. Dis. 189:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Castillo, I., E. Rodríguez-Iñigo, J. Bartolomé, S. de Lucas, N. Ortíz-Movilla, J. M. López-Alcorocho, M. Pardo, and V. Carreño. 2005. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut 54:682-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao, C. C., R. Jensen, and M. O. Dailey. 1997. Mechanisms of L-selectin regulation by activated T cells. J. Immunol. 159:1686-1694. [PubMed] [Google Scholar]
- 5.Cramp, M. E., S. Rossol, S. Chokshi, P. Carucci, R. Williams, and N. V. Naoumov. 2000. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 118:346-355. [DOI] [PubMed] [Google Scholar]
- 6.Day, C. L., G. M. Lauer, G. K. Robbins, B. McGovern, A. G. Wurcel, R. T. Gandhi, R. T. Chung, and B. D. Walker. 2002. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J. Virol. 76:12584-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, C. L., N. P. Seth, M. Lucas, H. Appel, L. Gauthier, G. M. Lauer, G. K. Robbins, Z. M. Szczepiorkowski, D. R. Casson, R. T. Chung, S. Bell, G. Harcourt, B. D. Walker, P. Klenerman, and K. W. Wucherpfennig. 2003. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Investig. 112:831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diepolder, H. M., J. T. Gerlach, R. Zachoval, R. M. Hoffmann, M. C. Jung, E. A. Wierenga, S. Scholz, T. Santantonio, M. Houghton, S. Southwood, A. Sette, and G. R. Pape. 1997. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J. Virol. 71:6011-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659-662. [DOI] [PubMed] [Google Scholar]
- 10.Hengel, R. L., V. Thaker, M. V. Pavlick, J. A. Metcalf, G. Dennis, Jr., J. Yang, R. A. Lempicki, I. Sereti, and H. C. Lane. 2003. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J. Immunol. 170:28-32. [DOI] [PubMed] [Google Scholar]
- 11.Hultgren, C., I. Desombere, G. Leroux-Roels, J. A. Quiroga, V. Carreño, B. Nilsson, O. Weiland, and M. Sallberg. 2004. Evidence for a relation between the viral load and genotype and hepatitis C virus-specific T cell responses. J. Hepatol. 40:971-978. [DOI] [PubMed] [Google Scholar]
- 12.Kamal, S. M., J. Fehr, B. Roesler, T. Peters, and J. W. Rasenack. 2002. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology 123:1070-1083. [DOI] [PubMed] [Google Scholar]
- 13.Kamal, S. M., A. Amin, M. Madwar, C. S. Graham, Q. He, A. Al Tawil, J. Rasenack, T. Nakano, B. Robertson, A. Ismail, and M. J. Koziel. 2004. Cellular immune responses in seronegative sexual contacts of acute hepatitis C patients. J. Virol. 78:12252-12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, D. E., K. Sugimoto, F. Ikeda, J. Stadanlick, M. Valiga, K. Shetty, K. R. Reddy, and K. M. Chang. 2005. T-cell response relative to genotype and ethnicity during antiviral therapy for chronic hepatitis C. Hepatology 41:1365-1375. [DOI] [PubMed] [Google Scholar]
- 15.Kodali, V. P., S. C. Gordon, A. L. Silverman, and D. G. McCray. 1994. Cryptogenic liver disease in the United States: further evidence for non-A, non-B, and non-C hepatitis. Am. J. Gastroenterol. 89:1836-1839. [PubMed] [Google Scholar]
- 16.Koziel, M. J., D. K. Wong, D. Dudley, M. Houghton, and B. D. Walker. 1997. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J. Infect. Dis. 176:859-866. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 18.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 19.Lauer, G. M., E. Barnes, M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, G. K. Robbins, D. R. Casson, M. Reiser, G. Dusheiko, T. M. Allen, R. T. Chung, B. D. Walker, and P. Klenerman. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924-936. [DOI] [PubMed] [Google Scholar]
- 20.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, W. M., J. E. Polson, D. S. Carney, B. Sahin, and M. Gale. 2005. Reemergence of hepatitis C virus after 8.5 years in a patient with hypogammaglobulinemia: evidence for an occult viral reservoir. J. Infect. Dis. 192:1088-1092. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Labrador, F. X., X. S. He, M. Berenguer, R. C. Cheung, T. L. Wright, and H. B. Greenberg. 2004. The use of class-I HLA tetramers for the detection of hepatitis C virus NS3-specific CD8(+) T cells in patients with chronic infection. J. Immunol. Methods 287:91-99. [DOI] [PubMed] [Google Scholar]
- 23.Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet 359:1478-1483. [DOI] [PubMed] [Google Scholar]
- 24.Pardo, M., J. M. López-Alcorocho, I. Castillo, E. Rodríguez-Iñigo, A. Perez-Mota, and V. Carreño. 2006. Effect of antiviral therapy for occult HCV infection. Aliment. Pharmacol. Ther. 23:1153-1159. [DOI] [PubMed] [Google Scholar]
- 25.Pardo, M., J. M. López-Alcorocho, E. Rodríguez-Iñigo, I. Castillo, and V. Carreño. Comparative study between occult hepatitis C virus infection and chronic hepatitis C. J. Viral Hepat., in press. [DOI] [PubMed]
- 26.Pawlotsky, J. M. 1999. Diagnostic tests for hepatitis C. J. Hepatol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 27.Pham, T. N., S. A. MacParland, P. M. Mulrooney, H. Cooksley, N. V. Naoumov, and T. I. Michalak. 2004. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J. Virol. 78:5867-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Post, J. J., Y. Pan, A. J. Freeman, C. E. Harvey, P. A. White, P. Palladinetti, P. S. Haber, G. Marinos, M. H. Levy, J. M. Kaldor, K. A. Dolan, R. A. French, A. R. Lloyd, W. D. Rawlinson, and Hepatitis C Incidence and Transmission in Prisons Study Group. 2004. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J. Infect. Dis. 189:1846-1855. [DOI] [PubMed] [Google Scholar]
- 28a.Quiroga, J. A., I. Castillo, E. Rodríguiz-Iñigo, M. Pardo, and V. Carreño. 2004. AASLD abstract. Abstract 259. Hepatology 40(Suppl. S4):278A. [Google Scholar]
- 29.Radkowski, M., A. Horban, J. F. Gallegos-Orozco, A. Pawelczyk, J. Jablonska, J. Wilkinson, D. Adair, and T. Laskus. 2005. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology 41:106-114. [DOI] [PubMed] [Google Scholar]
- 30.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215-229. [DOI] [PubMed] [Google Scholar]
- 31.Rico, M. A., J. A. Quiroga, D. Subirá, E. García, S. Castañón, M. Sällberg, G. Leroux-Roels, O. Weiland, M. Pardo, and V. Carreño. 2002. Features of the CD4+ T-cell response in liver and peripheral blood of hepatitis C virus-infected patients with persistently normal and abnormal alanine aminotransferase levels. J. Hepatol. 36:408-416. [DOI] [PubMed] [Google Scholar]
- 32.Rico, M. A., S. Ruiz, D. Subirá, G. Barril, S. Cigarrán, S. Castañón, J. A. Quiroga, R. Selgas, and V. Carreño. 2004. Virus-specific effector CD4+ T-cell responses in hemodialysis patients with hepatitis C virus infection. J. Med. Virol. 72:66-74. [DOI] [PubMed] [Google Scholar]
- 33.Rigby, S., and M. O. Dailey. 2000. Traffic of L-selectin-negative T cells to sites of inflammation. Eur. J. Immunol. 30:98-107. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Iñigo, E., J. M. López-Alcorocho, J. Bartolomé J., N. Ortiz-Movilla, M. Pardo, and V. Carreño. 2005. Percentage of hepatitis C virus-infected hepatocytes is a better predictor of response than serum viremia levels. J. Mol. Diagn. 7:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuer, P. J. 1991. Classification of chronic viral hepatitis: a need for reassessment. J. Hepatol. 13:372-374. [DOI] [PubMed] [Google Scholar]
- 36.Scognamiglio, P., D. Accapezzato, M. A. Casciaro, A. Cacciani, M. Artini, G. Bruno, M. L. Chircu, J. Sidney, S. Southwood, S. Abrignani, A. Sette, and V. Barnaba. 1999. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J. Immunol. 162:6681-6689. [PubMed] [Google Scholar]
- 37.Scriba, T. J., M. Purbhoo, C. L. Day, N. Robinson, S. Fidler, J. Fox. J. N. Weber, P. Klenerman, A. K. Sewell, and R. E. Phillips. 2005. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J. Immunol. 175:6334-6343. [DOI] [PubMed] [Google Scholar]
- 38.Semmo, N., E. Barnes, C. Taylor, J. Kurtz, G. Harcourt, N. Smith, and P. Klenerman. 2005. T-cell responses and previous exposure to hepatitis virus in indeterminate blood donors. Lancet 365:327-329. [DOI] [PubMed] [Google Scholar]
- 39.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strader, D. B., T. Wright, D. L. Thomas, L. B. Seeff, and American Association for the Study of Liver Diseases. 2004. Diagnosis, management, and treatment of hepatitis C. Hepatology 39:1147-1171. [DOI] [PubMed] [Google Scholar]
- 41.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, J. L., M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 42.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C., Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, A. R., H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]