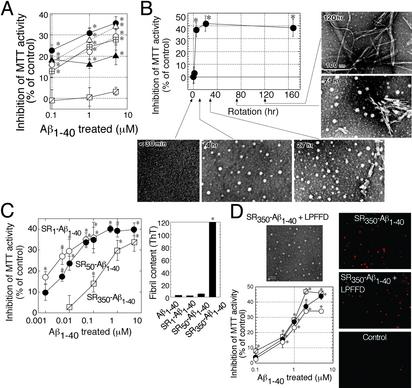
Fig. 1.
ASPD as a neurotoxin in Aβ1–40 aggregates. (A)Aβ1–40 solutions (350 μM) were aggregated, and their toxicity was estimated by MTT assay (n = 6). Aβ1–40 solution was rotated in Eppendorf tubes (•), glass tubes (▵), carbon-coated tubes (○), gold-coated tubes (⊞), and silicon-coated tubes (▴). Aβ1–40 solution without rotation also is shown ( ). Hereafter, we designated toxic Aβ1–40 aggregates formed by slowly rotating Aβ1–40 solution (350 μMin50% PBS) as SR350–Aβ1–40. (B) The structure and toxicity of Aβ1–40 aggregates formed by slow rotation. At the indicated time, aliquots were removed from SR350–Aβ1–40 for transmission electron microscopy and MTT assay (n = 9) (5 μM). (C) Aβ1–40 solutions [350 μM (SR350–Aβ1–40), 50 μM (SR50–Aβ1–40), and 1 μM (SR1–Aβ1–40)] each were rotated slowly for 7 days, and the toxicity was assessed (n = 7). Thioflavine T assay (20 μM; Sigma; excitation at 445 nm and emission at 485 nm; ref. 48) determined fibril content in freshly dissolved Aβ1–40 and in each SR–Aβ1–40 (n = 6). (D) SR350–Aβ1–40 was prepared with or without KLVFF or LPFFD. The toxicity was determined by MTT assay (n = 9) or propidium iodide staining (Right). Image of SR350–Aβ1–40 with LPFFD is shown (bar, 100 nm). Also shown are SR350–Aβ1–40 (○) with KLVFF (•) and with LPFFD (
). Hereafter, we designated toxic Aβ1–40 aggregates formed by slowly rotating Aβ1–40 solution (350 μMin50% PBS) as SR350–Aβ1–40. (B) The structure and toxicity of Aβ1–40 aggregates formed by slow rotation. At the indicated time, aliquots were removed from SR350–Aβ1–40 for transmission electron microscopy and MTT assay (n = 9) (5 μM). (C) Aβ1–40 solutions [350 μM (SR350–Aβ1–40), 50 μM (SR50–Aβ1–40), and 1 μM (SR1–Aβ1–40)] each were rotated slowly for 7 days, and the toxicity was assessed (n = 7). Thioflavine T assay (20 μM; Sigma; excitation at 445 nm and emission at 485 nm; ref. 48) determined fibril content in freshly dissolved Aβ1–40 and in each SR–Aβ1–40 (n = 6). (D) SR350–Aβ1–40 was prepared with or without KLVFF or LPFFD. The toxicity was determined by MTT assay (n = 9) or propidium iodide staining (Right). Image of SR350–Aβ1–40 with LPFFD is shown (bar, 100 nm). Also shown are SR350–Aβ1–40 (○) with KLVFF (•) and with LPFFD ( ). Data represent the mean ± SE. *, Significant difference from the control. The results suggest that toxicity of SR350–Aβ1–40 is associated with the spherical structures (B, 4 h), which we call ASPD.
). Data represent the mean ± SE. *, Significant difference from the control. The results suggest that toxicity of SR350–Aβ1–40 is associated with the spherical structures (B, 4 h), which we call ASPD.