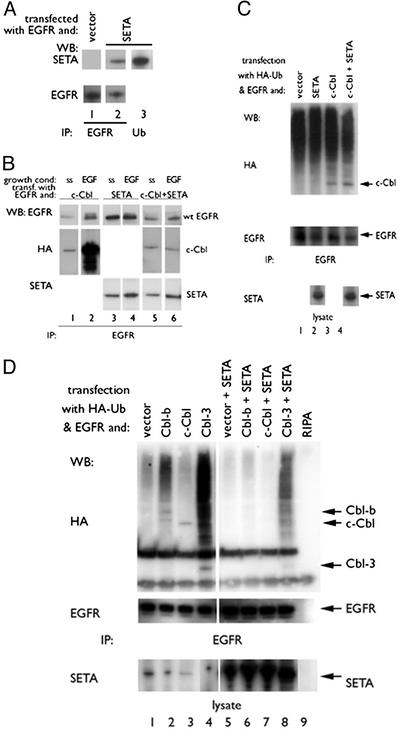
Fig. 1.
SETA regulates the Cbl–EGFR interaction. (A) In HEK293 cells, EGFR IP (lanes 1 and 2) identified SETA as part of the EGFR complex (lane 2). Antipolyubiquitin IPs contained SETA (lane 3). (B) In HEK293, cells were allowed to reach confluency, serum-starved (ss), and were then exposed to 100 ng/ml EGF for 5 min (EGF). c-Cbl proteins and SETA could be seen to move to the EGFR on EGF stimulation (lanes 1–4). Cotransfection of SETA abrogated the increased association of c-Cbl with the EGFR after stimulation (lanes 5 and 6). (C) In subconfluent HEK 293 cells, analysis of EGFR IP showed that the presence of c-Cbl and the levels of EGFR polyubiquitination, detected as a high-molecular-weight smear, were unaffected by the presence of SETA. (D). In contrast, in confluent HEK293 cells, analysis of EGFR IPs showed that SETA and Cbl proteins were present, and that SETA reduced the amount of EGFR-associated Cbls (compare lanes 2–4 with lanes 6–8), as well as EGFR polyubiquitination.