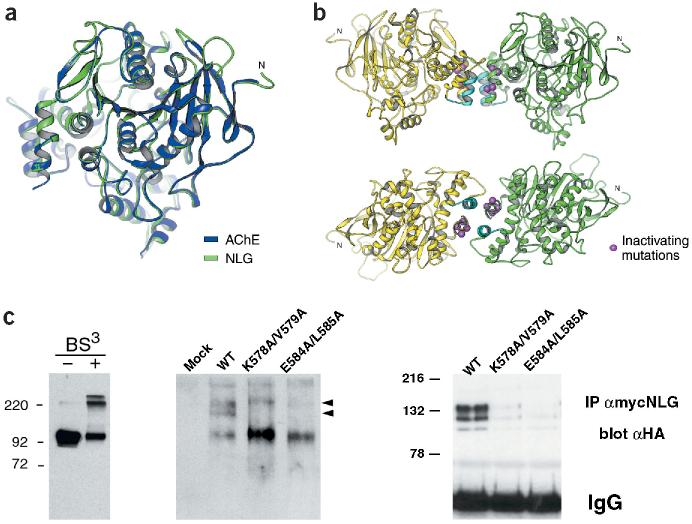
Figure 3.
Oligomerization of the neuroligin-1 AChE-homologous domain. (a) Mouse AChE crystal structure10 (blue) and modeled neuroligin-1 structure (green). Regions mutated in NLG/AChE-4 and NLG/AChE-6 are part of a four-helix bundle at the base of the AChE-homologous domain of neuroligin-1 (located at the left of the drawing). (b) In the crystal structure, AChE forms tetramers assembled from two dimers10. Two views of analogous, predicted neuroligin-1 dimers. Regions mutated in NLG/AChE-4 (highlighted in light blue) and NLG/AChE-6 are part of a four-helix bundle; residues E584/L585 and K578/V579 are marked in purple. (c) Analysis of neuroligin oligomers. Left panel, purified recombinant extracellular domain of neuroligin-1 could be cross-linked into dimers and tetramers. Higher-order complexes of wild-type neuroligin with different electrophoretic mobility were detected by native gel electrophoresis and western blotting (middle panel, arrowheads). Right panel, oligomerization of neuroligin was also detected by co-immunoprecipitation of differentially tagged neuroligin forms. Wild-type neuroligin was precipitated with myc antibodies and any co-precipitating HA-tagged wild-type or mutant neuroligin protein was detected by immunoblotting with anti-HA antibodies. Expression levels of all transfected constructs were monitored by western blotting of total cell lysates and were similar (data not shown and Supplementary Fig. 1).