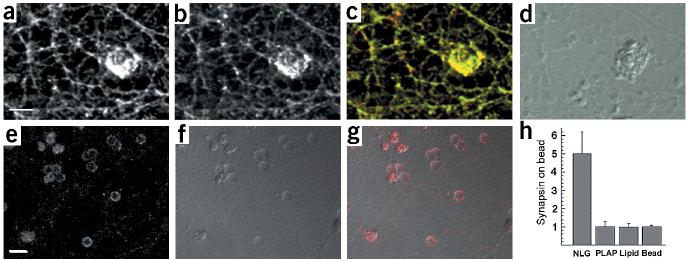
Figure 6.
Induction of neurexin clustering and presynaptic differentiation by purified neuroligin. (a–d) Beads coated with lipid bilayers containing purified GPI–neuroligin were added to 12-d.i.v. hippocampal cultures. After 24 h, cultures were fixed and stained with antibodies for neurexin (a, green in the overlay) and synaptobrevin (b, red in the overlay). (d) A differential interference contrast (DIC) image of the lipid-coated beads. Note that these beads coated with a lipid bilayer have a more irregular, rough surface, whereas uncoated beads (without lipid bilayer) appear as discreet particles with a smooth outline (see Supplementary Fig. 4 online for more details). (e–g) Uptake of synaptotagmin antibodies at axonal specializations induced by neuroligin-coated beads. Cultures were incubated with beads as above and depolarized in the presence of antibodies directed against the luminal domain of synaptotagmin. (e) Staining for bound synaptotagmin antibodies (red in the overlay). (f) A DIC image of the beads. (g) Overlay of synaptotagmin staining and DIC image. (h) Quantitation of synapsin clustering on silica beads. The average intensity of synapsin staining on the bead and in a neighboring area of equal size was measured and the enrichment of staining on beads over the neighboring area was calculated. The graph shows the mean ± s.d. (n = 10 beads each). Beads coated with GPI-anchored neuroligin-1 reconstituted into lipid bilayers (NLG) show a five-fold enrichment of synapsin staining. No enrichment is observed for beads coated with GPI-anchored placental alkaline phosphatase reconstituted into lipid bilayers (PLAP), beads coated only with lipid bilayers (lipid) or uncoated beads (bead). Scale bars are 5 μm (a) and 10 μm (e). See Supplementary Fig. 4 online for more details.