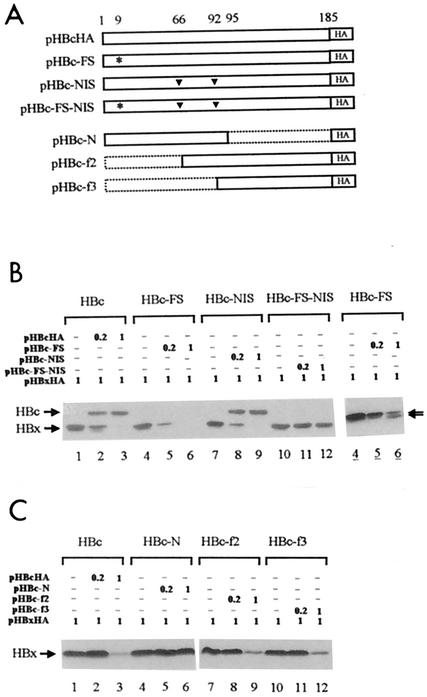
FIG. 3.
HBc protein is responsible for degradation of HBx. (A) Diagram of HBc mutants. Upper panel: HBc-FS had an additional nucleotide in codon 9, as indicated by the asterisk, preventing the sequence downstream from translating in frame. In HBc-NIS, two internal in-frame start codons at positions 66 and 92 were replaced by codons for Ile and Pro, respectively, as indicated by the inverted triangles. HBc-FS-NIS had a frameshift insertion and the two substitutions. Lower panel: HBc-N contained the N-terminal part of HBc from codons 1 to 95. HBc-f2 started with a Met codon at the 66th position and ended at the C terminus. Another Met codon at the 92nd position was not changed in this construct. HBc-f3 started with a Met codon at the 92nd position and ended at the C terminus. All constructs had an HA epitope at the C terminus. (B and C) Coexpression of HBx with HBc mutant proteins. The amount of transfected DNAs is indicated in micrograms above each lane. Lanes 4 to 6, longer exposure of the autoradiograph shown in B, lanes 4 to 6. The two smaller forms of HBc, very similar in size to HBx, are indicated by arrowheads.