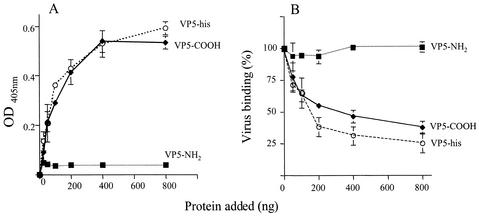
FIG. 5.
Effect of the two domains of VP5 on binding of rotaviruses to Hsc70. (A) ELISA plates coated with Hsc70 (500 ng/well) were incubated for 1 h at 37°C with increasing amounts of recombinant VP5-His, VP5-NH2, or VP5-COOH proteins, and the amount of recombinant protein bound to Hsc70 was detected with MAbs to VP5 as described in Materials and Methods. The amount of recombinant protein added to each well is plotted against the reading for optical density at 405 nm (OD405) obtained in the ELISA plate. (B) In a parallel plate, purified RRV virus particles (300 ng/well) were added after removing the unbound recombinant proteins, and the plate was further incubated for 1 h at 37°C. The amount of virus bound to Hsc70 was determined with an antibody to VP7 rotavirus protein (MAb 159). Data are expressed as the percentage of virus bound to Hsc70 when this protein was incubated with PBS as control. The arithmetic means and standard deviations for at least two independent experiments performed in duplicate are shown.