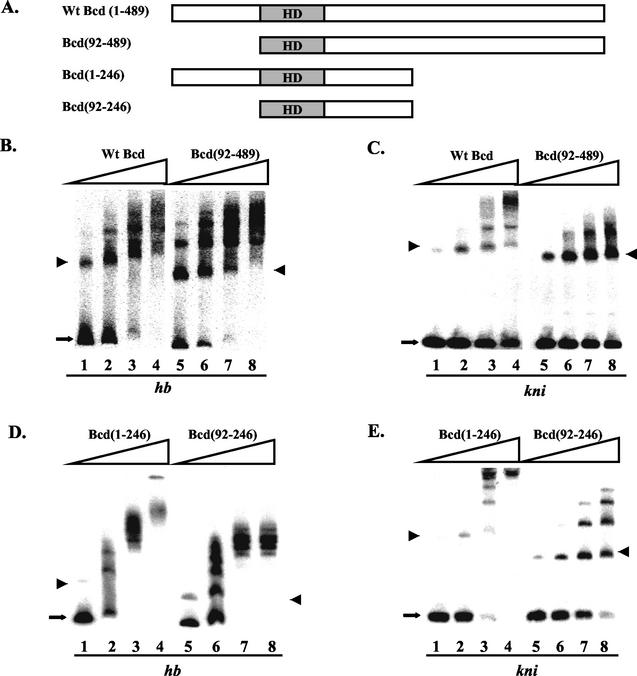
FIG. 2.
The amino-terminal domain of Bcd is preferentially required for cooperative DNA binding to the kni enhancer element. (A) Schematic diagrams showing the Bcd proteins used in gel shift assays. HD, homeodomain. (B to E) Gel shift assay results on hb (B and D) and kni (C and E) probes with the indicated proteins. For these experiments, the DNA probe concentration was 1.6 × 10−11 M. The proteins were normalized according to their specific activity measured on a single consensus Bcd binding site. The estimated active protein concentrations were 8 × 10−11 M (lanes 1 and 5), 1.6 10−10 M (lanes 2 and 6), 3.2 × 10−10 M (lanes 3 and 7), and 6.4 × 10−10 M (lanes 4 and 8). The monomeric complexes are marked with arrowheads, and the free probes are marked with arrows. The data shown in panels B to E were used in a Scatchard analysis to estimate Hill coefficient values. The estimated values on hb and kni elements were, respectively, 1.7 and 2.4 for wild-type Bcd, 1.3 and 1.0 for Bcd(92-489), 3.1 and 3.7 for Bcd(1-246), and 2.3 and 1.8 for Bcd(92-246). Although poor resolution of protein-DNA complexes in some gels (due to protein sizes) offered only limited accuracy, these estimated Hill coefficient values show that the removal of the amino-terminal domain preferentially reduces cooperative binding to the kni enhancer element (over twofold reduction in Hill coefficient) compared to that with the hb enhancer element (less than 1.4-fold reduction). See Fig. 6C and D and the Fig. 6 legend for further details. Wt, wild type.