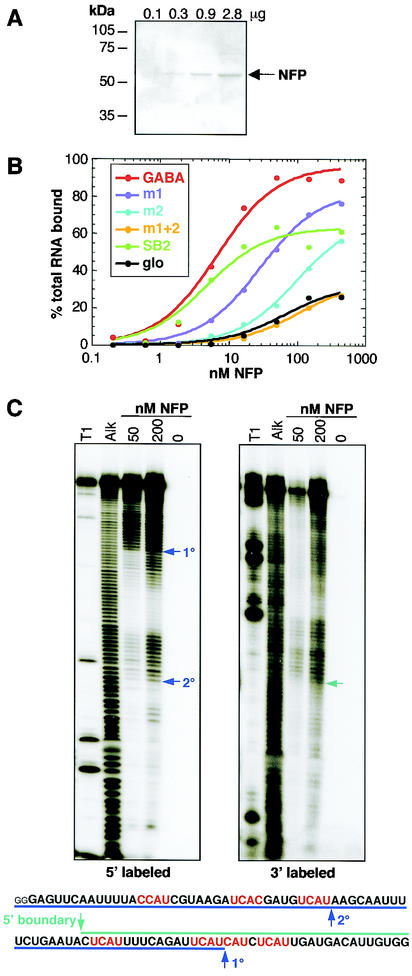
FIG. 6.
Nova-1 binds with high affinity to GABAARγ2 RNA in vitro. (A) His-tagged NFP was expressed in Escherichia coli and purified over a nickel column. Aliquots of increasing concentrations were analyzed by sodium dodecyl sulfate-PAGE followed by staining with Coomassie stain. (B) Nitrocellulose filter binding assays were performed using the NFP shown in panel A and six RNAs transcribed in vitro. GABA, RNA corresponding to the region of GABAARγ2 intron 8 highlighted in Fig. 5, with the addition of the sequences GGGAG at the 5′ end and CUAGCAAA at the 3′ end derived from the PCR primers used to amplify the template prior to in vitro transcription; m1 and m2, mutations of three and four YCAY repeats to YAAY in GABA, respectively; SB2, RNA obtained by RNA selection with Nova-1 (6); glo, RNA derived from human β-globin which spans regions of exon 1 and intron 1 and contains no YCAY elements. (C) Mapping the boundaries of Nova-1 binding to GABAARγ2 intron 9 RNA. RNA was labeled with 32P at either the 5′ or 3′ end and subjected to mild alkaline hydrolysis (Alk). The RNA was then incubated with NFP at a final concentration of 50 or 200 nM, and protein:RNA complexes werecaptured by filtration through nitrocellulose filters. Bound RNAs were eluted and analyzed by denaturing PAGE. RNase T1 digestion (T1) was used to generate size standards. Boundaries are highlighted by arrows and indicated on the transcribed RNA sequence shown at the bottom of each panel. 1°, primary boundary; 2°, secondary boundary. Small letters represent sequences derived from primers.