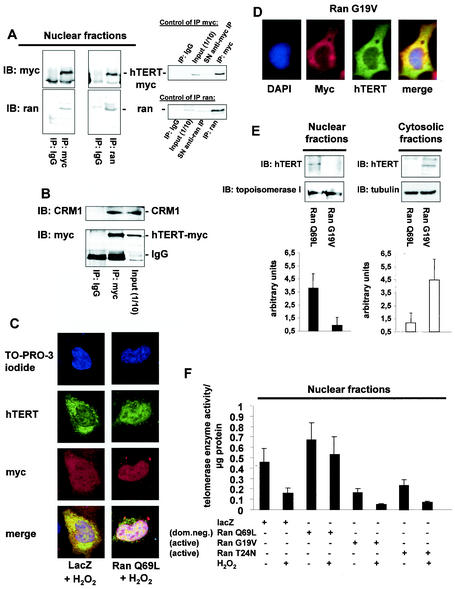
FIG. 4.
Nuclear export of hTERT is dependent on the GTPase Ran. (A) Lysates from 293 cells overexpressing myc-tagged, full-length hTERT were immunoprecipitated (IP) with an anti-myc antibody (left panels), an anti-Ran antibody, or an anti-mouse immunoglobulin G (IgG) antibody as indicated. Immunoblot (IB) analysis was performed against anti-Ran (lower panels) or anti-myc (upper panels) antibody. The right panels show the quality of the immunoprecipitations using an anti-myc or anti-Ran antibody. The input lane represents 1/10 of the cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) Lysates from 293 cells overexpressing myc-tagged hTERTwt were immunoprecipitated with an antimouse IgG or an anti-myc antibody as indicated, and immunoblot analysis was performed with an anti-CRM-1 antibody (upper panel). Membranes were stripped and reprobed with an anti-myc antibody (lower panel). The input lane represents 1/10 of the cell lysate used for immunoprecipitation. (C) A representative immunostaining is shown from cells overexpressing myc-tagged LacZ or myc-tagged Ran Q69L (dominant negative) incubated with H2O2 for 3 h. Cells were stained with TO-PRO-3 iodide to visualize the nuclei (blue, upper panel) and anti-TERT antibody followed by a goat anti-rabbit FITC-conjugated antibody (green, second upper panel) and anti-myc antibody (red, second lower panel). Cells were visualized by confocal microscopy. (D) A representative immunostaining is shown from cells overexpressing myc-tagged Ran G19V (active). Cells were stained with DAPI to visualize the nuclei (blue, left panel) and anti-TERT antibody followed by a biotin-labeled secondary antibody and streptavidin-FITC (green, second left panel) and anti-myc antibody (red, second right panel). (E) Nuclear and cytosolic lysates of cells overexpressing Ran Q69L or Ran G19V were dissolved by SDS-PAGE. Immunoblotting was performed with an antibody against hTERT (upper panels), and equal loading was confirmed either with topoisomerase 1 (nuclear fractions) or tubulin (cytosolic fractions) (n = 4) (middle panels). Densitometric analysis of four independent experiments is shown (lower panels). (F) Telomerase enzyme activity was measured in nuclear lysates of cells overexpressing LacZ, Ran Q69L, Ran G19V, or Ran T24N (n = 4) incubated in the presence or absence of H2O2 for 3 h.