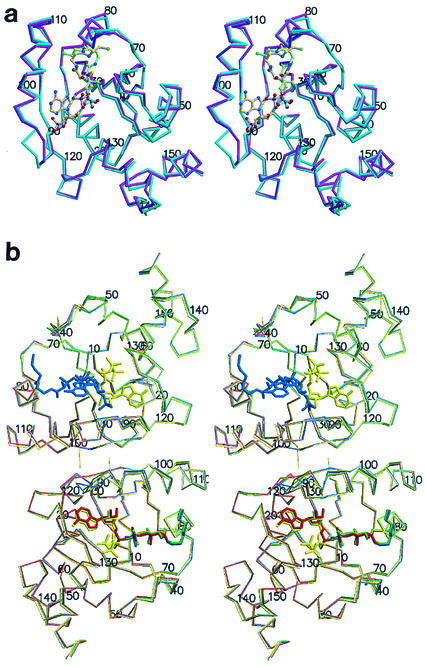
FIG. 2.
(a) Stereodiagram of the PPAT:CoA Cα trace of the two superimposed subunits within the asymmetric unit. Protomer A showing the entire inhibitor (magenta) and the twofold-related superimposed protomer B (teal) are shown. Ligands are shown in ball and stick representation. Oxygen (red) nitrogen (blue), sulfur (green), carbon (yellow), and phosphorus (black) atoms are shown. The overall rms deviation for the 314 equivalent Cα positions of the superposition of the two subunits in the asymmetric unit is 0.81 Å. This color coding of atoms is also used in panel b and in Fig. 3. (b) Stereodiagram of the PPAT Cα trace of the dimer within the asymmetric unit bound to CoA (blue), dPCoA (red) (Protein Data Bank identification code [PDB ID] 1B6T) (14), ATP (yellow) (PDB ID 1GN8) (16), and Ppant (green) (PDB ID 1QJC) (16). Protomer A (top subunit) binds CoA in the PPAT:CoA structure and ATP in the PPAT:ATP structure, while protomer B (bottom subunit) binds dPCoA (PPAT:dPCoA structure), ATP (PPAT:ATP structure), and Ppant (PPAT:Ppant structure) but shows only partially ordered binding to CoA (PPAT:CoA structure). This figure was produced with MolScript (19) or BobScript (11) and Raster3D (22) programs.