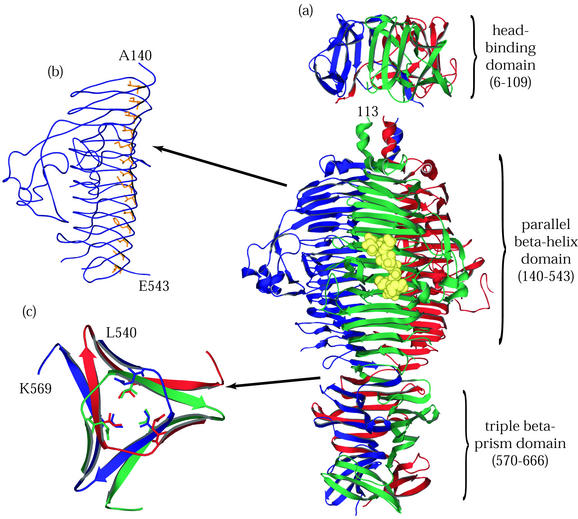
FIG. 3.
P22 tailspike protein. The illustrations are based on structures determined by Steinbacher and coworkers (59-62). (a) The entire P22 tailspike protein, shown bound to the nonasaccharide from S. enterica serovar 253Ty O-antigen (in yellow space-filling representation). The N-terminal domain is at the top, and the three subunit chains are shown in red, green, and blue. (b) An interior hydrophobic stack from one of the three identical single-chain, parallel β-helices is shown with side chains highlighted in yellow. (c) Residues 540 to 569, viewed from above and showing inwardly pointing hydrophobic residues. This region, which spans the interdigitated domain, forms one turn of a triple-stranded β-helix and is involved in trimer stability.