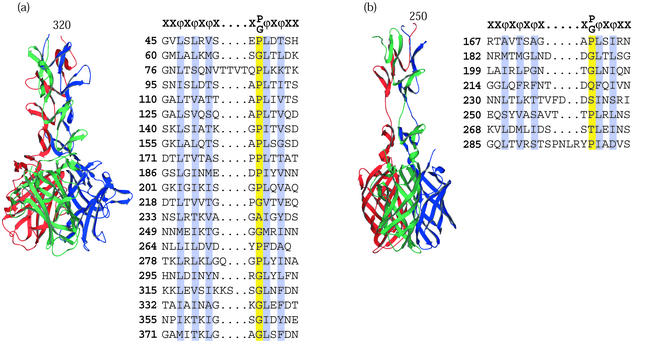
FIG. 4.
Viral attachment fibers that have triple β-spiral repeats. (a) Structure of the human Ad2 penton fiber (65) showing four triple β-spiral repeats (residues 320 to 392) in the N-terminal shaft domain and the CAR binding C-terminal knob (residues 399 to 582). (b) Model of the reovirus σ1 attachment protein structure (15) showing three triple β-spiral repeats (residues 246 to 309) and the C-terminal knob (residues 310 to 455). The two knob proximal repeats are separated by a flexible spacer. The knob is a β-barrel composed of two key motifs shown as Greek letters. Both complexes are shown with the N-terminal domains at the top and the chains colored red, green, and blue. For both proteins, alignments of previously defined 15-amino-acid repeats are shown, conserved hydrophobic or structural residues are highlighted, and a canonical repeat motif is given at the top of each alignment.