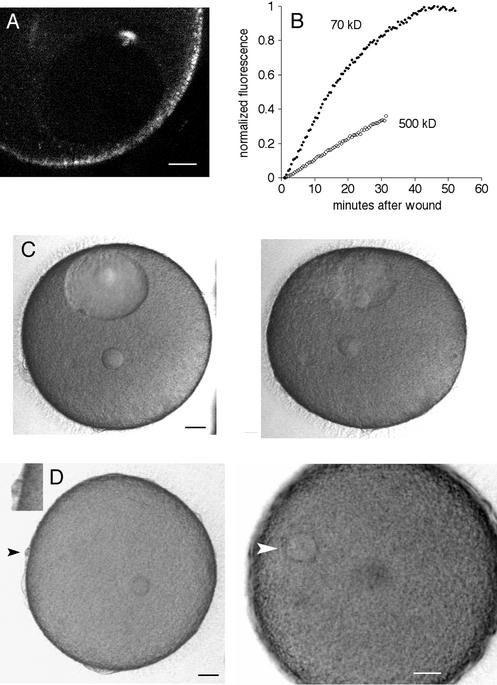
Figure 7.
Wounding of the nuclear envelope of starfish oocytes. (A) A multiphoton line scan was done across the nuclear envelope. This fluorescence image was obtained by multiphoton excitation at 800 nm. The nuclear interior has less autofluorescence than cytoplasm and is visible as the dark oval. The fluorescent scar is present at the site of the wound. The surface of the oocyte contains autofluorescent organelles. Scale bar, 20 μm. (B) Oocytes were injected in the cytoplasm with either rhodamine 70 kDa dextran or fluorescein 500 kDa dextran, and then the nuclear envelope was wounded by multiphoton line scan as above. The entry of fluorescence dextran in the nucleus was monitored. The fluorescence level at the start was set to 0 and was normalized to twice the starting cytoplasmic fluorescence because the nuclear space for soluble markers is about twice that in cytoplasm due to volume exclusion by yolk platelets (Terasaki, 1994). (C) Collapse of the nucleus after a line scan wound at the nuclear envelope. Images were taken every 30 s (see online move: collapse.mov). The first image, shown on the left, was taken 13 min after the wound. The image on the right was taken at 50 min, after the nucleus had collapsed. Scale bar, 20 μm. (D) Oocytes with a collapsed nucleus are still able to undergo meiotic divisions. The same oocyte shown in the previous panel was exposed to the maturation hormone 2.0 h after the nuclear envelope wound. Images were taken every 30 s beginning 2 min after application of the hormone (see online movie: division.mov). The left panel was taken at 91 min, soon after the first polar body had formed (black arrowhead; inset shows polar body at twice magnification). The small circle within the egg cytoplasm is the oil drop from the injection of 70 kDa rhodamine dextran, which helps in visualizing the nuclear envelope for wounding. The right panel was taken at 4.0 h and shows the egg pronucleus (white arrowhead). Scale bars, 20 μm.