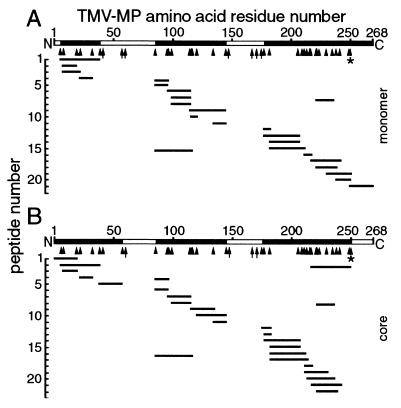
Figure 5.
MS of the full-length MP monomer (A) and core domain (B; Fig. 4). Molecular masses of peptides (short lines) generated by extensive trypsin digestion matched the predicted masses deduced from the MP sequence. Two hydrophobic peptides containing putative transmembrane domains (residues 39 or 58–85, and 145–175; open bars) were rarely detected. The core (B) was generated by release of residues 250–268 from the C terminus (open bar) by cleavage at highly sensitive K residues (*). Other K and R residues were more resistant to cleavage (arrowheads) and some were not cleaved (arrows). In some experiments, residues 1–6 of the monomer (A) were detected. The profiles are representative of MP from P1 and were similar for P2.