Abstract
Intracellular membrane fusion requires that membrane-bound soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins on both vesicle and target membranes form a highly specific complex necessary to bring the membranes close in space. Ykt6p is a yeast R-SNARE protein that has been implicated in retrograde transport to the cis-Golgi compartment. Ykt6p has been also been found to fractionate with vacuole membranes and participate in a vacuolar SNARE complex in homotypic vacuole fusion. To investigate the role of Ykt6p in membrane traffic to the vacuole we generated temperature-sensitive mutations in YKT6. One mutation produces an early Golgi block to secretion, and overexpression of the SNARE protein Sft1p suppresses the growth and secretion defects of this mutation. These results are consistent with Ykt6p and Sft1p participating in a SNARE complex associated with retrograde transport to the cis-Golgi. A second set of mutations in YKT6 specifically affects post-Golgi membrane traffic to the vacuole, and the effects of these mutations are not suppressed by Sft1p overexpression. Defects are seen in carboxypeptidase Y sorting, alkaline phosphatase transport, and aminopeptidase I delivery, and in one mutant, overexpression of the SNARE protein Nyv1p suppresses the alkaline phosphatase transport defect. By mutationally separating early and late requirements for Ykt6p, our findings have revealed that Ykt6p is a R-SNARE protein that functions directly in the three biosynthetic pathways to the vacuole.
INTRODUCTION
The spatial and temporal maintenance of membrane-bound compartments is crucial in eukaryotes. To preserve the composition of membrane organelles properly within cells, the transport of proteins and membrane between these compartments is required. This traffic is mediated by small membrane-bound vesicles that bud from one compartment and fuse with the next (Rothman, 1994). The molecular machineries regulating the processes by which vesicles are targeted to their destination are made up of proteins that fall into conserved families. Vesicle budding is mediated by cytosolic coat proteins (Schekman and Orci, 1996), and the subsequent docking and fusion of the vesicles demands a set of proteins called soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (Rothman and Warren, 1994).
Rothman and colleagues (Sollner et al., 1993) hypothesized that fusion is mediated by v-SNAREs located on the vesicle membrane and t-SNAREs on the target membrane. In vitro studies of SNARE complex formation, together with the crystal structure of a plasma membrane SNARE complex, have revealed that the conserved coiled-coil regions of the SNAREs form a parallel four-helix bundle (Hanson et al., 1997; Sutton et al., 1998). The central layer of the SNARE complex four-helix bundle (the “0” layer) is composed of three polar (glutamine or Q) and one positively charged (arginine or R) amino acid side chains, each donated by one of the four helices. In fact, SNARE complexes seem unable to accommodate more than one helix with a basic residue in the “0” layer (Ossig et al., 2000; Katz and Brennwald, 2000; Dilcher et al., 2001), supporting the general model of three Q-SNAREs and one R-SNARE in each fusion complex.
In addition to SNARE proteins, members of the Rab and the Sec1 families seem to function in every transport vesicle targeting and fusion step (Jiang and Ferro-Novick, 1994; Pevsner, 1996; Novick and Zerial, 1997). The Saccharomyces cerevisiae genome seems to encode eight recognizable t-SNARE proteins and two soluble N-ethylmaleimide-sensitive factor attachment protein-25 like proteins (Holthuis et al., 1998), nine recognizable v-SNAREs (Gotte and von Mollard, 1998), 11 Rab-like or Ypt proteins (Lazar et al., 1997), and four Sec1-like proteins (Gotte and von Mollard, 1998). Although the original SNARE hypothesis suggested high specificity for a v-SNARE and its cognate t-SNARE, many SNARE proteins are likely to function in multiple trafficking steps. The best-characterized examples are the yeast cis-Golgi syntaxin-like Q-SNARE Sed5p (Sogaard et al., 1994; Banfield et al., 1995; von Mollard et al., 1997) and the yeast Q-SNARE Vti1p (von Mollard et al., 1997; Fischer von Mollard and Stevens, 1999; Dilcher et al., 2001), which are each involved in at least three different SNARE complexes.
The presumed lack of specificity due to the apparent promiscuity of Q-SNAREs is better understood with the recent results of Rothman and colleagues (Fukuda et al., 2000; McNew et al., 2000; Parlati et al., 2002). These investigators have suggested that a t-SNARE complex is composed of a syntaxin-like Q-SNARE and two nonsyntaxin light chains and that this trimeric t-SNARE binds to a single v-SNARE on the vesicle membrane to form the SNARE fusion complex. Therefore, according to this model a given t-SNARE light chain (such as the Q-SNARE Vti1p) could participate in several SNARE complexes with different partners and not compromise specificity. However, v-SNAREs (which are usually R-SNAREs, with the exception of Sft1p; Parlati et al., 2002) are proposed to act alone on the vesicle membrane to direct vesicles to a specific compartment, and thus v-SNAREs are predicted to be highly specific for a particular t-SNARE trimer.
Many pathways to the yeast vacuole have been identified genetically and biochemically (Schekman, 1992; Bryant and Stevens, 1998; Wendland et al., 1998). Proteins such as carboxypeptidase Y (CPY) take a route from the late Golgi to the vacuole through an endosomal intermediate, the prevacuolar compartment (PVC). Other proteins such as alkaline phosphatase (ALP) follow an alternative route, which bypasses the PVC. A third class of proteins is transported to the cell surface and reaches the vacuole after endocytosis. Unlike other vacuolar proteins, aminopeptidase I (API) does not travel along the endoplasmic reticulum (ER) and Golgi pathway to reach the vacuole. Instead, API is synthesized in the cytosol and then transported to the vacuole through the cytoplasm-to-vacuole targeting (CVT) pathway (Kim and Klionsky, 2000).
Several SNARE proteins have been identified that function at a post-Golgi step in membrane traffic to the yeast vacuole: Pep12p, Vam3p, Vam7p, Vti1p, and possibly Ykt6p, the only R-SNARE among these proteins. Ykt6p is required for yeast cell viability and is essential for ER–Golgi transport (McNew et al., 1997); unlike other R-SNAREs, Ykt6p does not have a transmembrane domain, but its membrane localization is essential and is mediated by isoprenylation (McNew et al., 1997). Ykt6p was initially identified in a SNARE complex with the Golgi t-SNARE Sed5p (Sogaard et al., 1994) and subsequently found in a vacuolar SNARE complex, suggesting that Ykt6p plays a role in homotypic vacuole fusion (Ungermann et al., 1999). Ykt6p has also been implicated in post-Golgi transport steps because ykt6-1 mutant cells secrete the vacuolar protein CPY (Tsui and Banfield, 2000; Tochio et al., 2001), and overexpression of Ykt6p partially suppresses vacuolar targeting in vti1 mutants (Dilcher et al., 2001).
Herein, we report the isolation and characterization of a collection of temperature-sensitive ykt6 mutations that result in defects in multiple biosynthetic pathways to the vacuole without blocking membrane transport at an early Golgi transport step. By separating early and late requirements for YKT6, our findings indicate that Ykt6p is a R-SNARE protein that functions directly in the CPY, ALP, and CVT pathways to the vacuole. These results are particularly surprising because v-SNAREs/R-SNAREs would be expected to be highly specific for a particular t-SNARE complex.
MATERIALS AND METHODS
Materials
Enzymes used in DNA manipulations were from New England Biolabs (Beverly, MA) or Roche Diagnostics (Indianapolis, IN). Oligonucleotides were synthesized by Biosource (Camarillo, CA). Horseradish peroxidase-conjugated anti-rabbit polyclonal antibodies were obtained from Bio-Rad (Hercules, CA). [35S]-Express label and ECL solution were purchased from PerkinElmer Life Sciences (Boston, MA). Fixed Staphylococcus aureus cells (IgG Sorb) were obtained from the Enzyme Center (Malden, MA). Oxalyticase was from Enzogenetics (Corvallis, OR), and Zymolase 100T was from Seikagaku (Tokyo, Japan). All other reagents were of high-purity commercial grade.
Plasmid manipulations were carried out in the Escherichia coli strains XL1 Blue or XLII Blue by using standard media. Yeast strains grown in YEPD (1% yeast extract, 1% peptone, 2% dextrose) or SD (standard minimal medium) with appropriate supplements. To induce expression under the GAL1 promoter, 2% dextrose was replaced by 2% raffinose and 2% galactose.
Plasmid Construction and Generation of ykt6 Mutants
The plasmids used in this study are listed in Table 1. pAR3 was constructed by polymerase chain reaction (PCR) amplifying YKT6 gene by using the oligonucleotides YKT6-ORF-5 (5′-GTC-TCT-GGC-ACA-GTT-TGA-CTG-CG-3′) and YKT6-ORF-3 (5′-GTT-TCC-CTT-GCT-GTC-ATT-GGC-3′) and cloning it into SacI/EcoRI sites in pBluescript II KS+ (Stratagene, La Jolla, CA). A 1.28-kb YKT6 gene fragment digested with SacI/EcoRI from pAR3 was ligated into the same sites in pRS416, pRS313, and YEp352 to generate pAR5, pAR6, and pSRG84, respectively. The YKT6 gene disruption construct pAR4 was created by digesting pAR3 with XbaI/NsiI, removing ∼560 base pairs of the YKT6 open reading frame (ORF), and replacing it with XbaI/PstI-digested LEU2 gene fragment from pJJ250 (Sikorski and Hieter, 1989). pAR8 was generated by digesting pAR3 with NsiI, filling in and subcloning BamHI linker into the filled-in vector. pAR9 was derived from pAR8 by subcloning the SacI/EcoRI fragment, including NsiI fragment-deleted YKT6 into the SacI/EcoRI sites of pRS316.
Table 1.
Plasmids used in this study
| Plasmid | Description | Source |
|---|---|---|
| pAR3 | YKT6 gene in pBluescript II KS+ | This study |
| pAR4 | Plasmid containing ykt6Δ::LEU2 disruption cassette | This study |
| pAR5 | CEN-URA3 plasmid encoding Ykt6p | This study |
| pAR6 | CEN-HIS3 plasmid encoding Ykt6p | This study |
| pAR8 | C-terminal deleted (NsiI-NsiI) ykt6 in pBluescript II KS+ | This study |
| pAR9 | CEN-URA3 plasmid containing C-terminal deleted (NsiI-NsiI) ykt6 | This study |
| pAR11 | CEN-URA3 plasmid containing the ykt6-11 allele | This study |
| pAR12 | CEN-URA3 plasmid containing the ykt6-12 allele | This study |
| pAR13 | CEN-URA3 plasmid containing the ykt6-13 allele | This study |
| pGFP-ALP | 2μ-URA3 plasmid encoding GFP-ALP | (Cowles et al., 1997) |
| pSRG84 | 2μ-URA3 plasmid encoding Ykt6p | This study |
| pYK2 | 2μ-URA3 plasmid encoding Sft1p | This study |
| pYK8 | Integrating plasmid for ykt6-12 allele (loop in/loop out) | This study |
| pYK10 | Integrating plasmid containing HIS3 for ykt6-11 allele | This study |
| pYK11 | Integrating plasmid containing HIS3 for ykt6-13 allele | This study |
| pKEB74 | Plasmid containing vps4Δ::TRP1 disruption cassette | This study |
| pFvM137 | 2μ-URA3 plasmid encoding Nyv1p | (Fischer von Mollard and Stevens, 1999) |
PCR mutagenesis was carried out as described previously (Muhlrad et al., 1992; Burd et al., 1997) by using pAR3 as template and oligonucleotides complementary to plasmid sequences flanking the YKT6 gene to introduce mutations into the entire YKT6 sequence. To restrict mutations to sequences encoding the SNARE motif, ykt6-Nsi-5 (5′-GGA-TGA-ATA-TTT-AGT-CGC-ACA-TCC-3′) and ykt6-Nsi-3 (5′-TGC-TGC-TGT-CAT-TGG-CTT-TC-3′), were used to amplify a YKT6 C-terminal fragment in parallel reactions, by using either 25 mM MgCl2, 1 mM MnCl2, and 20 μM dATP, 250 μM dCTP, dGTP, and dTTP, or 20 μM dGTP, 250 μM dATP, dCTP, and dTTP to induce PCR errors. The 1.68-kb PCR product and EcoRI-digested pRS316, or the 0.295-kb PCR product and BamHI-digested pAR9, were cotransformed into ARY1. The transformants were plated on SD-URA media and screened for CPY secretion by colony overlay (Rothman and Stevens, 1986) and/or for growth at 37°C. Mutant plasmids were rescued and retested in ARY1. Plasmids conferring mutant phenotypes were sequenced at the Institute of Molecular Biology Biotechnology Laboratory (Eugene, OR). pAR11, pAR12, and pAR13 containing ykt6-11, ykt6-12, and ykt6-13, respectively, were chosen for further study. For integrating ykt6 mutants, pYK8 was produced by subcloning the 0.84-kb HindIII/EcoRI ykt6-12 fragment from pAR12 into the HindIII/EcoRI sites of YIp5 and then eliminating the 0.1-kb XbaI fragment. pYK10 and pYK11 were generated by ligation of the SacI/EcoRI fragments containing ykt6-11 and ykt6-13 from pAR11 and pAR13, respectively, into the SacI/EcoRI digested pRS303.
pYK2 was generated by subcloning the 1.2-kb KpnI/BglII fragment containing the SFT1 gene into the KpnI/BamHI sites in YEp352. pKEB74 was constructed by inserting the SalI/NotI vps4Δ::TRP1 fragment from pMB7 (Babst et al., 1997) into the same sites of pBluescript II KS+.
Yeast Strains
The strains used in this study are listed in Table 2. To construct a GAL1-YKT6 strain ARY1, the PCR-based gene deletion and modification method was used (Longtine et al., 1998). PCR product was generated using pFA6a-KanMX-PGAL1 as a template, and gal-ykt6–5 (5′-ATA-CAA-AAG-TCT-CTG-GCA-CAG-TTT-GAC-TGC-GTT-AGA-CCA-GGA-ATT-CGA-GCT-CGT-TTA-AAC-3′) and gal-ykt6–3 (5′-TTT-CTC-CTC-CAG-AGC-GAA-ATA-CAC-CGA-TGT-AGT-AGA-TTC-TCA-TTT-TGA–GAT-CCG-GGT-TTT-3′) as oligonucleotides. The PCR product was transformed into RPY10 to generate ARY1. The YKT6-disrupted strain ARY2 was generated by transforming SEY6210 with pAR5 as a covering plasmid and then transforming with SacI/XhoI-digested pAR4. Leu+ colonies were selected, and the deletion was confirmed by PCR. YKY5 was constructed by integration of XbaI-digested pYK8 into SEY6210 and looping out the wild-type YKT6 on 5-fluoroorotic acid plates (Boeke et al., 1984). YKY10 and YKY11 were derived from ARY2 by transforming pYK10 and pYK11, respectively, after linearizing with SnaBI. YKY12 was generated by transforming SalI/NotI-digested pKEB74 into SEY6210. YKY13 was derived from YKY10 by transforming SalI/NotI-digested pKEB74.
Table 2.
Yeast Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- | (Robinson et al., 1988) |
| RPY10 | MATα leu2-3,112ura3-52 his4-519 ade6 gal2 | (Piper et al., 1995) |
| ARY1 | MATα leu2-3,112ura3-52 his4-519 ade6 gal2 GAL1::YKT6 | This study |
| ARY2 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- ykt6::LEU2 + pAR5 | This study |
| YKY5 | MATtα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- ykt6-12 | This study |
| YKY10 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- ykt6-11::HIS3::ykt6Δ::LEU2 | This study |
| YKY11 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- ykt6-13::HIS3::ykt6Δ::LEU2 | This study |
| YKY12 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel-vps4Δ::TRP1 | This study |
| YKY13 | MATα leu2-3,112ura3-52 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ mel- ykt6-11::HIS3::ykt6Δ::LEU2 vps4Δ::TRP1 | This study |
Immunoblotting
Exponentially growing cells (10 OD600 total) were collected by centrifugation. Yeast extracts were prepared by resuspending the cells in 200 μl of 8 M Urea, 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, and 5% 2-mercaptoethanol. Glass beads were added and the samples were vortex mixed for 10 min. The extracts were subjected to SDS-PAGE (10% acrylamide gel). The proteins were transferred to nitrocellulose membrane and Ykt6p was visualized by incubation with polyclonal anti-Ykt6p antibody and horseradish peroxidase anti-rabbit secondary antibody followed by chemiluminescence.
Immunoprecipitation of 35S-labeled Proteins
Pulse-chase immunoprecipitation of radiolabeled CPY, ALP, API, Invertase, and Vps10p was performed essentially as described previously (Franzusoff and Schekman, 1989; Klionsky et al., 1992; Vater et al., 1992; Nothwehr et al., 1993; von Mollard et al., 1997). Yeast cultures were grown overnight at 25 or 30°C in synthetic minimal media lacking methionine to mid-log phase. Cells were then transferred to 1 OD600/ml in fresh media lacking methionine with 50 mM KPO4, pH 5.7, and 2 mg/ml bovine serum albumin and preincubated for 15 min at appropriate temperatures before pulse-labeling with [35S]-Express labeling mix (10 μl/0.5 OD unit of cells) for 10 min. The radiolabeled cells were chased for the indicated times by addition of unlabeled methionine and cysteine. The medium (extracellular fractions for CPY and invertase immunoprecipitation) was separated, and the cells were pelleted and spheroplasted using oxalyticase.
For CPY immunoprecipitations, the cells were lysed using 2% SDS. After incubation at 100°C for 5 min, the lysates and the supernatants were then adjusted to 0.1% SDS, 0.1% Triton X-100, 2 mM EDTA, and 90 mM Tris, pH 8.0. The fractions were precleared with S. aureus cells (IgG Sorb), incubated with anti-CPY serum (1 μl), and followed by a second incubation with IgG Sorb. Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. ALP and Vps10p immunoprecipitations were carried out as described above, except spheroplasts were lysed using 1% SDS, 8 M urea, and anti-ALP (2 μl) and anti-Vps10p (1 μl) sera were used for immunoprecipitations. For API immunoprecipitation, the spheroplasts were lysed in 1% SDS, 3 M urea, and 50 mM NaPO4, pH 7.0, and the resulting lysates were adjusted to 0.1% SDS, 0.5% Triton TX-100, 0.1 mM EDTA, 150 mM NaCl, and 50 mM Tris, pH 7.5. Anti-API serum (2 μl) was added to immunoprecipitate API. To induce invertase, the cells were incubated in synthetic minimal medium containing 0.1% dextrose, 50 mM KPO4, pH 5.7, and 2 mg/ml bovine serum albumin for 45 min at 25 and 30°C, or 30 min at 25°C plus 15 min at 37°C. After pulse and chase, the cells were pelleted and spheroplasted, and the medium was saved. The spheroplasts were pelleted and lysed in 2% SDS and 1× phosphate-buffered saline. The supernatant was combined with the medium to yield the extracellular fraction. The fractions were adjusted to 0.1% SDS, 1% Triton TX-100, and 1× phosphate-buffered saline before adding anti-invertase serum (2 μl) to immunoprecipitate invertase.
Suppressor Screen
To identify multicopy suppressors that allow temperature-sensitive ykt6 mutants to grow at the nonpermissive temperature, the strain YKY5 (ykt6-12) was transformed with a YEp24 2 μ library (Carlson and Botstein, 1982). Ts+ colonies that grew at 37°C were isolated and tested for the inability to grow on 5-fluoroorotic acid plates to determine whether the suppression was dependent on plasmids. Plasmids were recovered and retransformed into YKY5 to confirm the suppression. To determine the minimal DNA fragment required for suppression, portions of the inserts were subcloned into Yep352.
Fluorescence Microscopy
Cells were grown to early log phase in selective media at 30°C and examined using an Axioplan 2 fluorescence microscope (Carl Zeiss, Thornwood, NY) fitted with an Orca 100 digital camera (Hamamatsu, Bridgewater, NJ). Images were generated using Adobe Photoshop software.
RESULTS
Ykt6p Is Necessary for Proper Sorting of CPY and ALP
Ykt6p is an R-SNARE that is essential for yeast cell viability. McNew et al. (1997) placed the human homolog of YKT6 under the control of the GAL1 promoter and carried out a GAL shutoff experiment in ykt6Δ cells and found that an ER form p1CPY accumulated as the level of hYkt6p dropped, suggesting an ER-to-Golgi transport function for Ykt6p. However, this is exactly the same result we observed when we performed a GAL shutoff analysis of an essential Q-SNARE Vti1p, yet more extensive analysis involving vti1-ts mutants revealed that Vti1p is involved in directing multiple membrane traffic steps (von Mollard et al., 1997; Fischer von Mollard and Stevens, 1999). Ykt6p also has been shown to be a component of a vacuolar SNARE complex required for homotypic vacuolar fusion (Ungermann et al., 1999), and Ykt6p has been implicated in post-Golgi membrane traffic (Tsui and Banfield, 2000; Dilcher et al., 2001; Tochio et al., 2001). Based on these observations, we strongly suspected that Ykt6p might play important roles in post-Golgi membrane traffic.
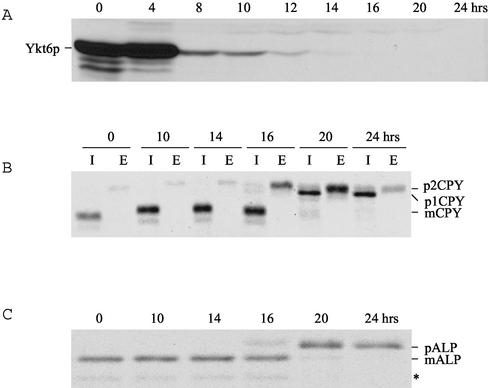
To examine the phenotypic consequences of the loss of endogenous Ykt6p, the YKT6 gene was placed under the control of GAL1 promoter. ykt6Δ cells carrying the GAL1-YKT6 gene were grown on galactose-containing medium and shifted to glucose-containing medium. The cells continued to grow up to 24 h after shifting from galactose-containing medium to glucose-containing medium (our unpublished data). Immunoblot analysis for Ykt6p showed that Ykt6p expression level under control of GAL1 promoter was ∼10-fold higher in galactose medium than under YKT6 promoter control in glucose medium and that the Ykt6p expression level approached the wild-type level ∼10 h after shift to glucose medium (Figure 1A). There was no detectable Ykt6p after 16 h after the shift to glucose medium.
Figure 1.
Depletion of Ykt6p results in Golgi and post-Golgi membrane transport blocks. (A) Ykt6p Western blot of whole cell extracts of GAL1-YKY6 strains (ARY1) grown in galactose and glucose media. The cultures were diluted when they reached an optical density of ∼1 to keep cells growing in logarithmic phase. Cells (10 OD) were collected at the indicated times and equivalent cell extracts were analyzed by SDS-PAGE and immunoblotting with antiserum against Ykt6p. (B) Strains were pulse labeled with [35S]methionine for 10 min at 30°C and chased for 45 min, and CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions and analyzed by SDS-PAGE and autoradiography. (C) Strains were labeled and chased as described above, and ALP was immunoprecipitated and the protein resolved by SDS-PAGE, and visualized by autoradiography. PEP4-dependent cleavage of proALP (pALP) results in the formation of the mature form (mALP) as well as an additional commonly observed degradation product (*).
To determine whether the depletion of Ykt6p affects transport to the vacuole, the processing of vacuolar hydrolases CPY and ALP was analyzed by pulse-chase immunoprecipitation experiments in GAL1-YKT6 cells grown on galactose medium or after shift to glucose medium for different periods of time. CPY and most other vacuolar proteins take a route from the trans-Golgi compartment to the vacuole through the PVC, whereas ALP reaches the vacuole through an alternative route, which bypasses the PVC (Conibear and Stevens, 1998). When cells were grown in galactose-containing medium, almost all of the CPY was found as its mature form (mCPY) in the cells, indicating proper CPY sorting and delivery of CPY to the vacuole. When depletion of Ykt6p was induced in glucose-containing medium, the Golgi-modified form p2CPY was secreted to the outside of cells after 16-h shift to glucose medium, implying that a post-Golgi transport step may be affected by the depletion of Ykt6p function (Figure 1B). The block in CPY transport to the vacuole was essentially complete after 20-h shift to glucose medium. By 20 h on glucose medium the ER and early Golgi form p1CPY accumulated in the cells, and some p2CPY was secreted. After 24-h shift, almost all CPY accumulated in the cells as the p1 form (Figure 1B), suggesting that transport through the Golgi was disrupted due to the loss of Ykt6p function. We also followed traffic along the ALP pathway and found that only the mature form of ALP was present in GAL1-YKT6 cells shifted for up to 14-h shift to on glucose medium, indicating normal transport of ALP to the vacuole. However, the precursor form of ALP started occurring in the cells after 16-h shift to glucose medium, and only the precursor form of ALP was detected in the cells after 20-h shift to glucose medium (Figure 1C). These data indicate that depletion of Ykt6p results in a block in ALP processing. It is not yet clear whether the ALP processing defect resulting from depletion of Ykt6p resulted from a disruption of ER-to-Golgi transport or a post-Golgi transport defect. Together, these data suggest that Ykt6p functions in more than one membrane transport pathway to the vacuole.
ykt6 Mutants Are Temperature Sensitive for Growth and Missort CPY
To address additional functions for Ykt6p in post-Golgi membrane traffic, we carried out a genetic analysis of its function. We generated point mutations in YKT6 on a CEN-based plasmid by error-prone PCR mutagenesis (Muhlrad et al., 1992; Burd et al., 1997) and screened for mutants exhibiting temperature-sensitive growth and for mutants that secret CPY. Plasmids were recovered, the YKT6 gene sequenced, and the ykt6 mutant alleles integrated into the genome for further analysis.
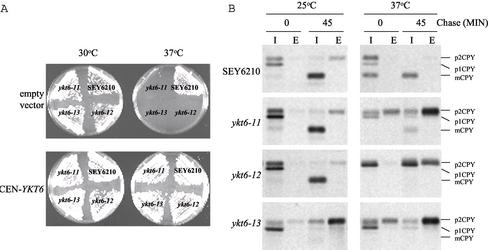
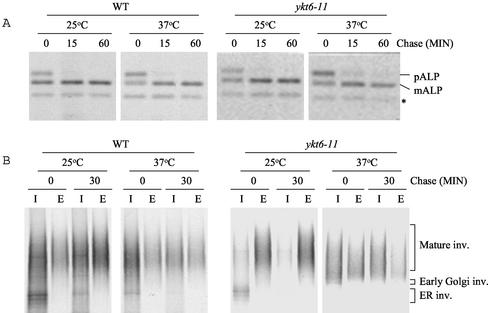
One mutant, ykt6-11, contained several mutations in YKT6 (Y5H, Y85H, K168E, K175Q, K184R, Q189R, and M200V), and although ykt6-11 cells grew normally at 30°C, they failed to grow at 37°C (Figure 2A). To follow the transport of newly synthesized CPY upon shift of the ykt6-11 mutant cells to 37°C, we used CPY pulse-chase immunoprecipitation. The majority of CPY reached the vacuole and was processed to mCPY when wild-type cells were 35S labeled at either 25 or 37°C. In ykt6-11 mutant cells, CPY was transported to the vacuole and processed normally at 25°C; however, the majority of CPY was secreted as the Golgi-modified p2CPY form at 37°C (Figure 2B). These data indicate that the ykt6-11 cells are defective in transport of CPY from the late-Golgi to the vacuole.
Figure 2.
ykt6 mutants are temperature sensitive for growth and CPY sorting. (A) Wild-type (SEY6210), ykt6-11 (YKY10), ykt6-12 (YKY5), and ykt6-13 (YKY11) cells were transformed with either a low copy plasmid carrying YKT6 (bottom) or an empty vector (top). The transformants were streaked on YEPD plates and incubated for3dat either 30°C (left) or 37°C (right). The growth defects are suppressed by the presence of the YKT6 plasmid. (B) Indicated strains were labeled with [35S]methionine for 10 min at 25°C or for 10 min at 37°C after a 15-min preincubation at 37°C, and chased for 45 min. CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions and analyzed by SDS-PAGE and autoradiography.
ykt6-12 mutant cells, which again contained multiple mutations in YKT6 (N117D, K143E, Q145H, N161D, and K175R), were also temperature sensitive for growth at 37°C (Figure 2A). In the ykt6-12 mutant cells, processing of CPY was normal at 25°C; however, upon shift to 37°C ∼60% of the CPY accumulated intracellularly and ∼40% was secreted as p2CPY (Figure 2B). There was no mature CPY present at the nonpermissive temperature in the ykt6-12 mutant cells, even after a 45-min chase period. These results indicate that in the ykt6-12 mutant cells, CPY accumulated either in the ER or in the cis-Golgi compartment at 37°C, and the CPY that did escape the early block was not transported to the vacuole but instead secreted as p2CPY. These data suggest that ykt6-12 mutant cells are blocked in transport at an early Golgi step as well as a Golgi to vacuole trafficking step at the nonpermissive temperature.
A third mutant allele of YKT6 was isolated (ykt6-13), and this allele contained the following point mutations: K143N, E160G, Q164R, F186L, and K188 M. ykt6-13 mutant cells grew somewhat slower at the lower temperature (25 and 30°C), but were completely growth defective at 37°C (Figure 2A). Interestingly, the ykt6-13 mutant cells secreted the majority of CPY as p2CPY even at 25°C (Figure 2B), reflecting a constitutive defect in post-Golgi CPY transport to the vacuole. Together, analysis of the new ykt6-ts mutants indicates that Ykt6p plays an important role in Golgi to vacuole transport along the CPY pathway.
ykt6-12 Cells Exhibit a cis-Golgi Transport Block at the Nonpermissive Temperature
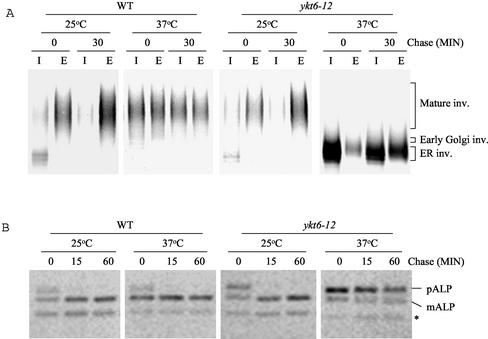
As shown above (Figure 2B), CPY pulse-chase immunoprecipitation experiments revealed that CPY accumulated in the ER or in the cis-Golgi compartment at the nonpermissive temperature in ykt6-12 mutant cells. To further test whether the ykt6-12 temperature-sensitive mutant is blocked in transport to the cis-Golgi compartment, the transport of the secretory protein invertase was followed by pulse-chase immunoprecipitation (Figure 3A). At the permissive temperature the core glycosylated ER form of invertase was precipitated from intracellular extracts of wild-type and ykt6-12 mutant cells before the chase, and fully glycosylated invertase was found in the medium after 30 min of chase at 25°C, indicating that secretion is normal in ykt6-12 cells at 25°C. At the restrictive temperature ykt6-12 mutant cells accumulated the core-glycosylated ER and early Golgi forms of invertase intracellularly even after 30 min of chase at 37°C. Invertase that escaped the ykt6-12 cis-Golgi block was secreted in a severely underglycosylated form. These data suggest that at the nonpermissive temperature ykt6-12 cells are defective in a cis-Golgi membrane traffic, and that outer-chain glycosylation in the Golgi apparatus is compromised.
Figure 3.
ykt6 cells are defective for invertase and ALP transport at the nonpermissive temperature. (A) Wild-type (SEY6210) and ykt6-12 (YKY5) strains containing a plasmid encoding invertase (pFvM104) were grown in low-glucose (0.1%) medium at 25°C to induce invertase expression. The strains were labeled with [35S]methionine for 10 min at 25°C or for 10 min at 37°C after a 15-min preincubation at 37°C and chased for 30 min. Invertase was immunoprecipitated from intracellular (I) and extracellular (E) fractions, separated by SDS-PAGE, and visualized by autoradiography. (B) Wild-type (SEY6210) and ykt6-12 (YKY5) strains were radiolabeled for 10 min at 25°C or for 10 min at 37°C after a 15-min preincubation at 37°C. After the indicated times, ALP was immunoprecipitated from cell extracts, and analyzed as described above. In addition to pALP and mALP, commonly observed degradation product (*) is indicated.
To examine the effect of the ykt6-12 mutation on the ALP pathway, ALP was immunoprecipitated from wild-type and ykt6-12 mutant cells after 35S labeling (Figure 3B). As expected, ALP was transported rapidly to the vacuole in wild-type cells at both 25 and 37°C, as revealed by its processing to the mature form (mALP). However, in ykt6-12 cells shifted to 37°C, ALP processing was severely blocked. This block in ALP processing could reflect a requirement for Ykt6p in post-Golgi ALP transport, or alternatively could reflect the cis-Golgi block in ykt6-12 cells. Further experiments would be required to implicate Ykt6p involvement in post-Golgi membrane traffic along the ALP pathway (see below).
SFT1 Overexpression Suppresses the ykt6-12 Mutation
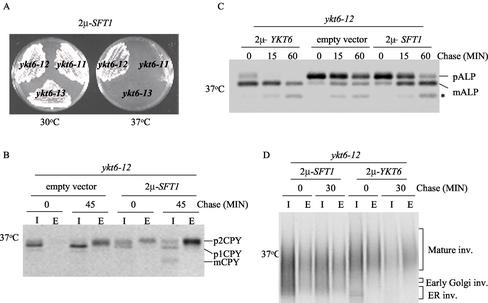
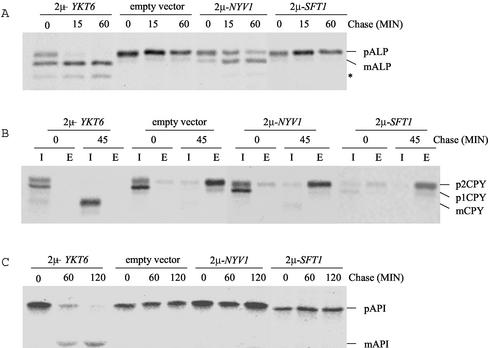
To further characterize the function of YKT6 in yeast membrane trafficking, we screened for multicopy suppressors of the 37°C growth defect of ykt6 mutants. ykt6-12 mutant cells were transformed with a multicopy yeast library (Carlson and Botstein, 1982) and colonies selected for growth at 37°C. The library plasmids were recovered from the transformants and retransformed into ykt6-12 mutant cells to confirm that suppression was due to the library plasmid. A library plasmid that allowed the ykt6-12 mutant cells to grow at 37°C was identified from this screen, and the minimal suppressing fragment contained the SFT1 gene. Sft1p is a SNARE protein involved in retrograde transport within the Golgi compartment (Banfield et al., 1995; Wooding and Pelham, 1998; Parlati et al., 2002). Suppression by Sft1p was allele specific, because overexpression of Sft1p allowed ykt6-12 cells to grow at 37°C, but did not suppress the growth defect of either ykt6-11 or ykt6-13 mutant strains (Figure 4A).
Figure 4.
Overexpression of Sft1p suppresses the temperature-sensitive growth and early Golgi transport defects of ykt6-12 cells. (A) ykt6-11 (YKY10), ykt6-12 (YKY5), and ykt6-13 (YKY11) cells carrying a multicopy plasmid with SFT1, streaked on YEPD plates, and incubated at either 30 or 37°C. (B–D) ykt6-12 (YKY5) cells were transformed with an empty vector, or a multicopy plasmid carrying either the YKT6 or SFT1 gene. These cells were labeled with [35S]methionine for 10 min after a 15-min preincubation at 37°C. After the indicated times, CPY, ALP, and invertase were immunoprecipitated as in Figure 3. I, intracellular; E, extracellular; *, ALP degradation product.
We tested the effect of overexpression of Sft1p on trafficking of CPY in the ykt6 mutants. Overexpression of Sft1p did not suppress the CPY sorting defect in ykt6-11 or ykt6-13 cells (see below). However, overexpression of Sft1p in ykt6-12 mutant cells almost completely alleviated the accumulation of p1CPY at the nonpermissive temperature, resulting in most of the CPY being secreted as the Golgi-modified p2CPY form (Figure 4B). These data suggest that ykt6-12 mutants are defective in at least two distinct steps in CPY sorting: transport beyond the cis-Golgi compartment and from the trans-Golgi to the vacuole, and that overexpression of Sft1p only enhances CPY transport through the early Golgi.
We also determined whether the ALP processing and invertase secretion defects in the ykt6-12 mutant could be suppressed by overexpression of Sft1p. Although overexpression of Sft1p did not completely restore ALP processing to normal in the ykt6-12 mutant at the restrictive temperature, a significant amount of ALP was delivered to the vacuole and processed by 60 min of chase (Figure 4C). Overexpression of Sft1p also suppressed the invertase defect in ykt6-12 cells (Figure 4D). These data further support the proposal that Ykt6p functions in cis-Golgi membrane traffic.
The ykt6-11 Mutation Blocks the CPY Pathway Downstream of the PVC
Analysis of ykt6-11 mutant cells demonstrated that the late Golgi form of CPY was secreted by these cells after a shift to the restrictive temperature (Figure 2B). In contrast, analysis of ALP and invertase revealed that the ykt6-11 mutant had no defect in ALP transport and processing, or in invertase secretion (Figure 5). These data suggest that ykt6-11 mutant cells have only a late Golgi or post-Golgi CPY pathway sorting defect.
Figure 5.
ykt6-11 cells are normal for transport of ALP and secretion of invertase. (A) Wild-type (SEY6210) and ykt6-11 (YKY10) strains were labeled and chased for the indicated times. The cell extracts were subjected to immunoprecipitation with antibodies to ALP, and the samples were analyzed by SDS-PAGE and autoradiography. (B) Wild-type (SEY6210) and ykt6-11 (YKY10) strains containing a plasmid containing SUC2 (pFvM104) were grown in low-glucose (0.1%) medium at 25°C to induce invertase expression. The strains were labeled with [35S]methionine for 10 min at 25°C or for 10 min at 37°C after a 15-min preincubation at 37°C, and chased for 30 min. Invertase was immunoprecipitated from intracellular (I) and extracellular (E) fractions, and analyzed as described for Figure 3. The degradation product of ALP is indicated (*).
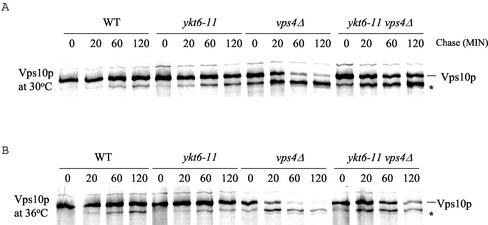
CPY is transported to the vacuole by a receptor-mediated process. The receptor is encoded by the VPS10 gene (Marcusson et al., 1994), and upon binding in the late Golgi, the ligand-receptor complex travels to the PVC. In the PVC, Vps10p dissociates from CPY and is recycled to the Golgi, whereas CPY is transported to the vacuole. Vps10p is very stable in wild-type cells, and mutations that prevent recycling from the PVC back to the Golgi result in the delivery of Vps10p to the vacuole where it undergoes proteolysis. We found that Vps10p was not exposed to vacuolar proteases in ykt6-11 cells shifted to 36°C, as evidenced by very little proteolysis of Vps10p even after a 2-h chase period (Figure 6). In ykt6-11 mutants shifted to 36°C, Vps10p may become trapped in vesicles that are unable to fuse with either the PVC or with the vacuole, causing a depletion of Vps10p from the Golgi and resulting in secretion of CPY.
Figure 6.
ykt6-11 cells are defective for CPY sorting at a step after the prevacuolar compartment. Wild-type (SEY6210), ykt6-11 (YKY10), vps4Δ (YKY12), and ykt6-11 vps4Δ (YKY13) cells were labeled with [35S]methionine for 10 min at 30°C (A) or 10 min at 36°C (B) after a 15-min preincubation at 36°C, and chased for the indicated times. Cell extracts were subjected to immunoprecipitation with antibodies to Vps10p. The samples were analyzed by SDS-PAGE and autoradiography. The Vps10p proteolytic product is indicated (*).
To determine whether the sorting block in the ykt6-11 mutant takes place upstream or downstream of the PVC, we carried out an epistasis experiment with the class E vps4Δ mutation (Babst et al., 1997). A large collection of vacuolar protein sorting (vps) mutants has been divided into six classes (A–F) on the basis of vacuolar morphology (Raymond et al., 1992). All class E vps mutants accumulate an exaggerated form of the PVC due to a membrane traffic block from the PVC to both the Golgi and the vacuole (Piper et al., 1995; Babst et al., 1997; Bryant and Stevens, 1997). This aberrant PVC contains active proteases, and therefore Vps10p that reaches the PVC undergoes proteolytic cleavage much more rapidly than in wild-type cells (Piper et al., 1995). VPS4 is a class E VPS gene whose product is an AAA–type ATPase, which is required for protein transport from the PVC to both the vacuole and Golgi (Babst et al., 1997). Mutant vps4 cells accumulate vacuolar, endocytic, and late Golgi membrane proteins in the aberrant PVC. We introduced the vps4 disruption mutation into wild-type cells and ykt6-11 mutants, and followed the fate of Vps10p in vps4Δ mutants and vps4Δ ykt6-11 double mutants at the permissive and nonpermissive temperatures. We used 36°C for the nonpermissive temperature instead of 37°C because Vps10p seemed particularly unstable in vps4Δ cells at 37°C. ykt6-11 mutant cells secreted the vast majority of CPY as the Golgi-modified p2CPY at 36°C just as at 37°C (Figure 2B; our unpublished data). The fate of Vps10p in wild-type, ykt6-11, vps4 and vps4Δ ykt6-11 cells was followed by immunoprecipitation of Vps10p before and after shift to the high temperature. Whereas Vps10p was stable in wild-type and ykt6-11 mutant cells, Vps10p in vps4Δ cells and vps4 Δykt6-11 double mutant cells was quickly subjected to proteolysis at both the low and high temperatures (Figure 6). It is likely that the slightly increased stability of Vps10p in the double mutant relative to vps4Δ cells reflects the higher level of secretion of vacuolar proteases due to the ykt6 mutation, and thus lower levels of proteases in the PVC of the double mutant. These data suggest that the CPY sorting defect in ykt6-11 cells occurs in membrane traffic between the PVC and the vacuole.
ykt6-13 Mutant Cells Are Defective in Multiple Vacuolar Pathways that Converge at the Vacuole
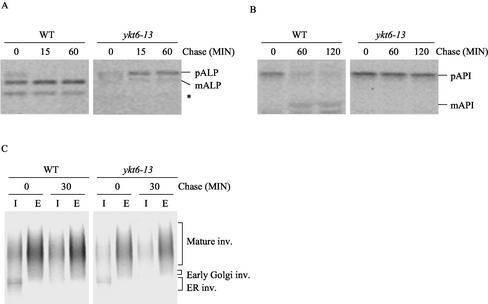
Although ykt6-13 cells were temperature sensitive for growth, these cells secreted the p2 form of CPY even at 25°C (Figure 2). In contrast, ykt6-13 cells exhibited normal secretion of invertase even at higher temperature (Figure 7C). These data suggest that the ykt6-13 mutant is normal for ER-to-Golgi membrane trafficking, but defective in CPY transport from the late Golgi compartment to the vacuole. Because ykt6-13 cells were temperature sensitive for growth but constitutively defective in CPY transport to the vacuole, we reasoned that ykt6-13 cells might also be defective for some other trafficking pathways to the vacuole in addition to the CPY pathway. To test whether Ykt6p is required for additional trafficking steps to the vacuole, trafficking of ALP and API in the ykt6-13 mutant was studied by pulse-chase immunoprecipitation experiments. Wild-type and ykt6-13 cells were labeled at 25, 30, or at 37°C after a 15-min shift to the labeling temperature, and ALP was immunoprecipitated after the indicated chase period (Figure 7A). Whereas ALP was transported to the vacuole and processed properly in wild-type cells, ALP processing in the ykt6-13 cells was completely blocked at all temperatures (Figure 7A). These data indicate that Ykt6p is required for transport of ALP to the vacuole.
Figure 7.
ykt6-13 cells exhibit defects in ALP and API transport to the vacuole, but are normal for secretion. (A and B) Wild-type (SEY6210) and ykt6-13 (YKY11) cells were labeled with [35S]methionine for 10 min at 30°C, and chased for the indicated times. ALP or API was immunoprecipitated from cell extracts and analyzed by SDS-PAGE and autoradiography. (C) Wild-type (SEY6210) and ykt6-11 (YKY10) cells containing the SUC2 plasmid (pFvM104) were grown in low-glucose (0.1%) medium at 25°C to induce invertase expression. The strains were labeled with [35S]methionine for 10 min at 30°C, and chased for 30 min. Invertase was immunoprecipitated from intracellular (I) and extracellular (E) fractions, and analyzed as described in Figure 3. The ALP degradation product is indicated (*).
Unlike other vacuolar proteins, API does not travel along the ER-Golgi pathway to reach the vacuole but alternatively, API follows the CVT pathway (Klionsky, 1998). API is synthesized in the cytosol and sequestered by double-membrane vesicles that fuse with the vacuole. API processing was analyzed in wild-type and ykt6-13 cells after labeling at 25 or 30°C. API was immunoprecipitated after 60 or 120 min of chase and resolved by SDS-PAGE. In wild-type cells, the majority of API was processed to the mature form of API after the 120-min chase period. However, processing of API was blocked in ykt6-13 cells at all temperatures (Figure 7B), suggesting that Ykt6p is essential for API transport to the vacuole. Interestingly, API transport was not disrupted in either ykt6-11 or ykt6-12 cells (our unpublished data). Together, the data from the ykt6-13 mutant indicate that whereas secretion is normal, traffic along all three pathways to the vacuole (CPY, ALP, and CVT) is blocked, suggesting that ykt6-13 cells may be defective in the final step of all of these pathways (i.e., membrane fusion with the vacuole).
Overexpression of Nyv1p Specifically Suppresses the ALP Trafficking Defect of ykt6-13 Cells
To further study the role of Ykt6p in the vacuolar transport pathways, we investigated whether overexpression of different SNARE proteins could suppress the defects in CPY, ALP, and API transport to the vacuole in ykt6 mutants. Interestingly, overexpression of Nyv1p restored the ALP transport defect in the ykt6-13 cells (Figure 8A). Nyv1p is a vacuolar R-SNARE, which has been identified in a vacuolar SNARE complex and implicated in functioning in homotypic vacuolar fusion steps (Nichols et al., 1997; Ungermann et al., 1999; McNew et al., 2000; Fukuda et al., 2000). Suppression of the ykt6-13 ALP pathway defect by Nyv1p was specific to this SNARE protein, because overexpression of other SNARE proteins (such as Sft1p) did not suppress the ALP transport defect (Figure 8A; our unpublished data). The suppression of ALP processing in ykt6 mutants by Nyv1p was allele specific, because overexpression of Nyv1p suppressed the ALP transport defect in ykt6-13 but not ykt6-12 cells (Figure 8A; our unpublished data). Whereas overexpression of Nyv1p suppressed the ALP transport defect in ykt6-13 cells, increased levels of Nyv1p had no effect on the CPY sorting and API transport defects of the ykt6-13 mutant (Figure 8, B and C). These observations suggest that Nyv1p may be able to replace Ykt6p in SNARE complex formation along the ALP pathway, but not along either the CPY or CVT pathways.
Figure 8.
Overexpression of Nyv1p suppresses the ALP trafficking defect of the ykt6-13 mutant. ykt6-13 (YKY11) cells carrying an empty plasmid or a multicopy plasmid with either YKT6, NYV1, or SFT1 were labeled for 10 min at 30°C and chased for the indicated times. Cell extracts were subjected to immunoprecipitation with antibodies to ALP (A), CPY (B), or API (C), and the samples resolved by SDS-PAGE and visualized by autoradiography. I, intracellular; E, extracellular; *, ALP degradation product.
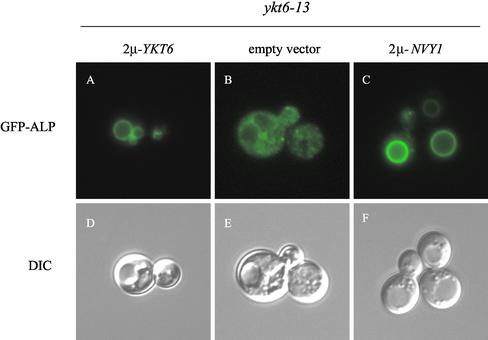
The ALP transport defect in ykt6-13 mutant cells was further investigated by monitoring the localization of green fluorescent protein (GFP)-ALP (Figure 9). GFP-ALP was localized to the vacuole membrane in cells expressing wild-type Ykt6p (Figure 9A), with the vacuole being apparent when viewed by Nomarski optics (Figure 9D). In ykt6-13 cells, GFP-ALP was found in a number of tiny spots through the cytoplasm may correspond to vesicles, and it was clear from the Nomarski images that GFP-ALP had not reached the vacuole membrane (Figure 9, B and E). Because overexpression of Nyv1p suppressed the ALP processing defect in ykt6-13 cells, we investigated the localization of GFP-ALP in ykt6-13 cells expressing high levels of Nyv1p. As shown in Figure 9, GFP-ALP was localized to the vacuole membrane in ykt6-13 cells overexpressing Nyv1p. These data indicate that the ALP processing defect observed in ykt6-13 cells (Figure 7A) is the result of a transport defect, and that high levels of Nyv1p somehow overcomes the defect in the fusion of ALP pathway membranes with the vacuole, possibly by replacing Ykt6p in the vacuole SNARE complex.
Figure 9.
GFP-ALP mislocalization in ykt6-13 cells is overcome by overexpression of Nyv1p. ykt6-13 (YKY11) cells carrying a plasmid expressing GFP-ALP were transformed with an empty, or a multicopy plasmid with either YKT6 or NYV1. GFP-ALP was visualized by fluorescence microscopy after cell growth at 30°C.
DISCUSSION
Ykt6p is an S. cerevisiae R-SNARE protein that is essential for yeast cell viability, and as cells are depleted for Ykt6p they become blocked along the secretory pathway at an early Golgi stage. Herein, we report the isolation of temperature-sensitive mutations in Ykt6p that affect a number of different membrane traffic steps in yeast. These data support a model in which Ykt6p functions in membrane traffic at the early Golgi compartment as well as three separate membrane transport pathways to the vacuole: the CPY, ALP, and CVT pathways. Therefore, the yeast R-SNARE Ykt6p, like the yeast Q-SNARE protein Vti1p, functions to promote membrane fusion in multiple membrane traffic pathways.
Role of Ykt6p in Vesicle Fusion at the cis-Golgi
Ykt6p was originally identified as a component of a cis-Golgi SNARE complex composed of Sed5p, Sec22p, Bet1p, Bos1p, Sft1p, and Gos1p in yeast cells (Sogaard et al., 1994; McNew et al., 1997). Ykt6p has been found to form tetrameric SNARE complexes with Sed5p, Tlg1p, and Vti1p, as well as with Sed5p, Gos1p, and Sft1p (Tsui et al., 2001), and Ykt6p functions specifically in an in vitro membrane fusion assay with the SNARE partners Sed5p, Gos1p and Sft1p (Parlati et al., 2002). Depletion of Ykt6p revealed that varying the level of this SNARE protein resulted in defects in membrane traffic at several steps along the secretory pathway. A significant defect in the sorting of CPY to the vacuole was observed at moderate levels of Ykt6p depletion (this study), whereas a block in the secretory pathway at an early Golgi step was observed after Ykt6p was depleted to very low levels (McNew et al., 1997; this study). Characterization of a new ykt6 temperature-sensitive mutant (ykt6-12) also supports a role for Ykt6p at an early Golgi step. We found that ykt6-12 cells accumulated ER and early Golgi forms of CPY, ALP, and invertase when shifted to the restrictive temperature. Interestingly, the high-temperature growth defect and the early Golgi transport block were suppressed by overexpression of the SNARE protein Sft1p, but not other SNAREs. It is likely that the increased levels of Sft1p help drive formation of the cis-Golgi SNARE complex with the functionally compromised Ykt6-12p. Suppression was specific to the early Golgi block, because ykt6-12 cells overexpressing Sft1p secreted CPY at the high temperature.
The phenotypes of ykt6 mutants described herein together with the presence of Ykt6p in Sed5p cis-Golgi SNARE complexes provide compelling evidence that Ykt6p functions in a membrane fusion step at the early Golgi compartment in yeast (Figure 10). Determining whether Ykt6p functions in anterograde or retrograde vesicle transport to the early Golgi has not been so clear. The R-SNARE Sec22p functions in anterograde ER-to-Golgi vesicle traffic together with the Q-SNAREs Sed5p, Bet1p, and Bos1p (McNew et al., 2000; Sacher et al., 1997; Stone et al., 1997). Overexpression of Ykt6p suppresses the growth defects of sec22 and bos1 mutations at the restrictive temperature (McNew et al., 1997), and recent data indicate that Ykt6p can functionally substitute for Sec22p in ER-to-Golgi transport (Liu and Barlowe, 2002). Together, these data indicate that the primary R-SNARE for ER-to-Golgi vesicle fusion is Sec22p.
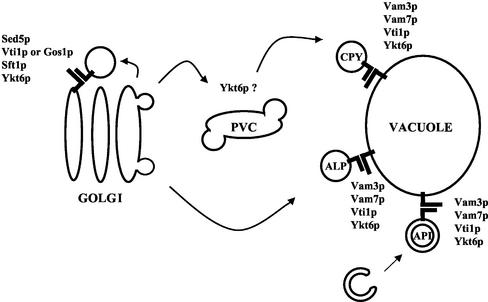
Figure 10.
Ykt6p functions in at least two different SNARE complexes in four membrane traffic pathways in yeast. Ykt6p forms a SNARE complex with Sed5p, Sft1p, and Vti1p (or Gos1p) in retrograde transport to the cis-Golgi compartment. Ykt6p also functions in SNARE complex formation with Vam3p, Vam7p, and Vti1p in the CPY, ALP and API transport pathways to the vacuole. Ykt6p may also function in vesicle fusion with the PVC (Dilcher et al., 2001).
It is likely that the normal role for Ykt6p in the early secretory pathway is in retrograde transport to the cis-Golgi, because it has been found that defects in retrograde transport lead to rapid loss of anterograde traffic (Lewis and Pelham, 1996). Therefore, the early Golgi transport block in our ykt6-12 mutant may result from a defect in retrograde transport to this compartment. This notion is supported by the observation that the growth and transport defects of ykt6-12 cells are suppressed specifically by overexpression of the retrograde SNARE Sft1p. In addition, a retrograde Q-SNARE Vti1p binds to Ykt6p, and overexpression of Ykt6p rescues vti1 temperature-sensitive mutants (Lupashin et al., 1997). Therefore, the available data favor a model in which the R-SNARE Ykt6p functions in retrograde transport to the cis-Golgi together with the SNARE proteins Sed5p, Sft1p, and either Vti1p or Gos1p. According to this model, Ykt6p could function in anterograde ER-to-Golgi transport when overexpressed or when yeast cells lack the nonessential ER-to-Golgi R-SNARE Sec22p (Liu and Barlowe, 2002).
Role of Ykt6p in CPY Sorting to the Vacuole
Whereas depletion of Ykt6p eventually led to an early Golgi transport block, at earlier times these yeast cells secreted the Golgi-modified form of CPY, suggesting that Ykt6p is required for post-Golgi membrane traffic to the vacuole. This view was supported by the isolation of temperature-sensitive mutations in the YKT6 gene that resulted in a number of post-Golgi, vacuole protein sorting defects at the restrictive temperature. Each of these temperature-sensitive mutations in YKT6 interfered with CPY sorting, causing these cells to secrete the Golgi-modified precursor form of CPY (p2CPY) at the restrictive temperature, and one of these mutants (ykt6-13) secreted CPY at all temperatures. In fact, ykt6-12 cells, which are blocked at an early Golgi transport step at 37°C, continued to secrete CPY even when they overexpressed Sft1p and the Golgi block was relieved. These data indicate that Ykt6p plays a crucial role in vesicle fusion at one of several possible steps in the CPY transport pathway to the vacuole (Figure 10).
It has been demonstrated previously that Vti1p binds to the prevacuolar Q-SNARE Pep12p and functions in transport from the trans-Golgi compartment to the PVC (von Mollard et al., 1997; Fischer von Mollard and Stevens, 1999). Results from suppression studies also suggest that Ykt6p functions with Vti1p and Pep12p in transport to the PVC (Dilcher et al., 2001). However, our epistasis experiment with the class E mutant vps4Δ reveals that the CPY sorting defect resulting from the ykt6-11 mutation occurs downstream of the PVC. Interestingly, overexpression of Pep12p did not suppress the missorting defects of any of our ykt6 mutants. This result is consistent with the epistasis experiment with the class E vps mutant, and indicates that the ykt6 mutations reported here disrupt the transport of CPY from the PVC to the vacuole. Therefore, the in vivo data indicate that the R-SNARE Ykt6p is very likely to play a role in vesicle fusion both at the PVC (Dilcher et al., 2001) and at a post-PVC step (this study; Figure 10).
Role of Ykt6p in Heterotypic Vacuole Fusion
A number of studies have suggested that Ykt6p may play a role in membrane fusion at the vacuole. Ykt6p is found in a SNARE complex with known vacuole SNARE proteins, and Ykt6p antibodies inhibit homotypic vacuole fusion in vitro (Ungermann et al., 1999). In addition, high-level expression of Ykt6p in vti1ts cells partially suppressed the defect in delivery of proteins along both the ALP and CVT pathways (Dilcher et al., 2001), suggesting that Ykt6p and Vti1p participate in SNARE complexes at the vacuole membrane. Our mutational analysis of YKT6 reveals that ykt6-13 mutant cells are defective for CPY sorting, API processing (CVT pathway) and for vacuolar delivery and processing of ALP. Taken together, these data make a compelling case that Ykt6p participates in membrane fusion at very late steps in each of the three membrane transport pathways leading to the vacuole, the ALP, CPY, and CVT pathways (Figure 10).
Previous studies using temperature-sensitive yeast mutants have shown that membrane transport along the CVT and ALP pathways requires the three Q-SNAREs Vti1p (Fischer von-Mollard and Stevens, 1999), Vam3p (Darsow et al., 1997), and Vam7p (Sato et al., 1998). In vitro studies revealed that these three Q-SNAREs form a t-SNARE complex that when incorporated into liposomes is functional for fusion with liposomes containing the R-SNARE Nyv1p (Fukuda et al., 2000). Interestingly, whereas Ykt6p could compete with Nyv1p for binding to the tripartite t-SNARE and inhibit Nyv1p-dependent liposome fusion, Ykt6p could not serve as the v-SNARE in this in vitro fusion assay. These in vitro results are in sharp contrast to the in vivo functional studies on Nyv1p and Ykt6p function, which revealed that yeast cells lacking Nyv1p have been found to be completely normal for all three biosynthetic pathways to the vacuole (ALP, CPY, and CVT; Nichols et al., 1997; Fischer von-Mollard and Stevens, 1999). The in vivo functional data (Tsui and Banfield, 2000; Dilcher et al., 2001; this study) argue that Ykt6p serves as the R-SNARE (and likely the v-SNARE) at the vacuole for the three biosynthetic pathways leading to this compartment.
Whereas Ykt6p functions in heterotypic fusion at the vacuole, all of the available data argue that the function of Nyv1p is restricted to homotypic fusion. However, we found that overexpression of Nyv1p suppressed the ALP trafficking defects of ykt6-13 mutant cells, suggesting that Nyv1p could substitute for Ykt6p along the ALP pathway. Interestingly, Nyv1p overexpression did not suppress the CPY or CVT pathway defects of ykt6-13 mutant cells. This pathway-specific effect could reflect the fact that Nyv1p is transported to the vacuole along the ALP pathway (Reggiori et al., 2000), such that Nyv1p is only present in ALP-containing vesicles.
The failure of Ykt6p to serve in vitro as the v-SNARE when combined with membranes containing the vacuolar t-SNARE (Vam3p/Vam7p/Vti1p) could reflect the fact that Ykt6p is tethered to the membrane by a C20 lipid chain rather than a transmembrane domain (Fukuda et al., 2000). Interestingly, McNew et al. (2000) reported that Ykt6p could serve as a v-SNARE coupled with the plasma membrane t-SNARE (Sso1p/Sec9p) when Ykt6p was anchored to the membrane through an artificial membrane-spanning peptide, leading these authors to conclude that the lipid anchor prevents Ykt6p from functioning as a v-SNARE. However, it is also possible that native lipid-anchored Ykt6p can only function as a v-SNARE in vivo, because all of the identified docking/tethering and other factors (Whyte and Munro, 2002) that might be needed to promote membrane fusion with Ykt6p as a v-SNARE were absent from the in vitro liposome fusion reactions. It will be interesting to learn whether addition of some of the functionally identified non-SNARE proteins can promote in vitro membrane fusion with the lipid-anchored Ykt6p v-SNARE and vacuolar t-SNARE combination.
Acknowledgments
We thank Scott Emr, Susan Ferro-Novick, Hugh Pelham, and Gerry Waters for providing plasmids. We also thank Daniel Klionsky for providing anti-API antibody and William Wickner for providing anti-Ykt6p antibody. We are grateful to all members of the Stevens laboratory for helpful discussions. This work was supported by National Institutes of Health grant GM-32448 (to T.H.S.) and a fellowship from the American Heart Association, Northwest Affiliate (to Y.K.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-10-0687. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0687.
References
- Babst, M., Sato, T.K., Banta, L.M., and Emr, S.D. (1997). Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16, 1820–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield, D.K., Lewis, M.J., and Pelham, H.R. (1995). A SNARE-like protein required for traffic through the Golgi complex. Nature 375, 806–809. [DOI] [PubMed] [Google Scholar]
- Boeke, J.D., LaCroute, F., and Fink, G.R. (1984). A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197, 345–346. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., and Stevens, T.H. (1997). Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J. Cell Biol. 136, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N.J., and Stevens, T.H. (1998). Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. Mol. Biol. Rev. 62, 230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C.G., Peterson, M., Cowles, C.R., and Emr, S.D. (1997). A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell. 8, 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M., and Botstein, D. (1982). Two differentially regulated mRNAs with different 5′ ends encode secreted with intracellular forms of yeast invertase. Cell 28, 145–154. [DOI] [PubMed] [Google Scholar]
- Conibear, E., and Stevens, T.H. (1998). Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404, 211–230. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., Odorizzi, G., Payne, G.S., and S.D. Emr. (1997). The A.P-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 91, 109–118. [DOI] [PubMed] [Google Scholar]
- Darsow, T., Rieder, S.E., and Emr, S.D. (1997). A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138, 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilcher, M., Kohler, B., and von Mollard, G.F. (2001). Genetic interactions with the yeast Q-SNARE VTI1 reveal novel functions for the R-SNARE YKT6. J. Biol. Chem. 276, 34537–34544. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard, G., and Stevens, T.H. (1999). The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell. 10, 1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff, A., and Schekman, R. (1989). Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 8, 2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, R., McNew, J.A., Weber, T., Parlati, F., Engel, T., Nickel, W., Rothman, J.E., and Sollner, T.H. (2000). Functional architecture of an intracellular membrane t-SNARE. Nature 407, 198–202. [DOI] [PubMed] [Google Scholar]
- Gotte, M., and von Mollard, G.F. (1998). A new beat for the SNARE drum [see comments]. Trends Cell Biol. 8, 215–218. [DOI] [PubMed] [Google Scholar]
- Hanson, P.I., Roth, R., Morisaki, H., Jahn, R., and Heuser, J.E. (1997). Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Holthuis, J.C., Nichols, B.J., Dhruvakumar, S., and Pelham, H.R. (1998). Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., and Ferro-Novick, S. (1994). Identification of yeast component A: reconstitution of the geranylgeranyltransferase that modifies Ypt1p and Sec4p. Proc. Natl. Acad. Sci. USA 91, 4377–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, L., and Brennwald, P. (2000). Testing the 3Q:1R “rule”: mutational analysis of the ionic “zero”layer in the yeast exocytic SNARE complex reveals no requirement for arginine. Mol. Biol. Cell 11, 3849–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., and Klionsky, D.J. (2000). Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu. Rev. Biochem. 69, 303–342. [DOI] [PubMed] [Google Scholar]
- Klionsky, D.J. (1998). Nonclassical protein sorting to the yeast vacuole. J. Biol. Chem. 273, 10807–10810. [DOI] [PubMed] [Google Scholar]
- Klionsky, D.J., Cueva, R., and Yaver, D.S. (1992). Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J. Cell Biol. 119, 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar, T., Gotte, M., and Gallwitz, D. (1997). Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem. Sci. 22, 468–472. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R. (1996). SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell 85, 205–215. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and Barlowe, C. (2002). Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol. Biol. Cell 13, 3314–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A., 3rd, Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998) Additional modules for versatile and economical P.C.R-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Lupashin, V.V., Pokrovskaya, I.D., McNew, J.A., and Waters, M.G. (1997). Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol. Biol. Cell 8, 2659–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson, E.G., Horazdovsky, B.F., Cereghino, J.L., Gharakhanian, E., and Emr, S.D. (1994). The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77, 579–586. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Parlati, F., Fukuda, R., Johnston, R.J., Paz, K., Paumet, F., Sollner, T.H., and Rothman, J.E. (2000). Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Sogaard, M., Lampen, N.M., Machida, S., Ye, R.R., Lacomis, L., Tempst, P., Rothman, J.E., and Sollner, T.H. (1997). Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J. Biol. Chem. 272, 17776–17783. [DOI] [PubMed] [Google Scholar]
- Muhlrad, D., Hunter, R., and Parker, R. (1992). A rapid method for localized mutagenesis of yeast genes. Yeast 8, 79–82. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., Ungermann, C., Pelham, H.R., Wickner, W.T., and Haas, A. (1997). Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387, 199–202. [DOI] [PubMed] [Google Scholar]
- Nothwehr, S.F., Roberts, C.J., and Stevens, T.H. (1993). Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J. Cell Biol. 121, 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., and Zerial, M. (1997). The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496–504. [DOI] [PubMed] [Google Scholar]
- Ossig, R., Schmitt, H.D., de Groot, B., Riedel, D., Keranen, S., Ronne, H., Grubmuller, H., and Jahn, R. (2000). Exocytosis requires asymmetry in the central layer of the SNARE complex. EMBO J. 19, 6000–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati, F., Varlamov, O., Paz, K., McNew, J.A., Hurtado, D., Sollner, T.H., and Rothman, J.E. (2002). Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA 99, 5424–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner, J. (1996). The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal. J. Neurosci. Res. 45, 89–95. [DOI] [PubMed] [Google Scholar]
- Piper, R.C., Cooper, A.A., Yang, H., and Stevens, T.H. (1995). VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, C.K., Howald-Stevenson, I., Vater, C.A., and Stevens, T.H. (1992). Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori, F., Black, M.W., and Pelham, H.R. (2000). Polar transmembrane domains target proteins to the interior of the yeast vacuole. Mol. Biol. Cell 11, 3737–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J.S., Klionsky, D.J., Banta, L.M., and Emr, S.D. (1988). Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J.E. (1994). Intracellular membrane fusion. Adv. Second Messenger Phosphoprotein Res. 29, 81–96. [DOI] [PubMed] [Google Scholar]
- Rothman, J.H., and Stevens, T.H. (1986). Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 47, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and Warren, G. (1994). Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 4, 220–233. [DOI] [PubMed] [Google Scholar]
- Sacher, M., Stone, S., and Ferro-Novick, S. (1997). The synaptobrevin-related domains of Bos1p and Sec22p bind to the syntaxin-like region of Sed5p. J. Biol. Chem. 272, 17134–17138. [DOI] [PubMed] [Google Scholar]
- Sato, T.K., Darsow, T., and Emr, S.D. (1998). Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol. Cell. Biol. 18, 5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman, R. (1992). Genetic and biochemical analysis of vesicular traffic in yeast. Curr. Opin. Cell Biol. 4, 587–592. [DOI] [PubMed] [Google Scholar]
- Schekman, R., and Orci, L. (1996). Coat proteins and vesicle budding. Science 271, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard, M., Tani, K., Ye, R.R., Geromanos, S., Tempst, P., Kirchhausen, T., Rothman, J.E., and Sollner, T. (1994). A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78, 937–948. [DOI] [PubMed] [Google Scholar]
- Sollner, T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P., and Rothman, J.E. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Stone, S., Sacher, M., Mao, Y., Carr, C., Lyons, P., Quinn, A.M., and Ferro-Novick, S. (1997). Bet1p activates the v-SNARE Bos1p. Mol. Biol. Cell 8, 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, R.B., Fasshauer, D., Jahn, R., and Brunger, A.T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Tochio, H., Tsui, M.M., Banfield, D.K., and Zhang, M. (2001). An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293, 698–702. [DOI] [PubMed] [Google Scholar]
- Tsui, M.M., and Banfield, D.K. (2000). Yeast Golgi SNARE interactions are promiscuous. J. Cell Sci. 113, 145–152. [DOI] [PubMed] [Google Scholar]
- Tsui, M.M., Tai, W.C., and Banfield, D.K. (2001). Selective formation of Sed5p-containing SNARE complexes is mediated by combinatorial binding interactions. Mol. Biol. Cell 12, 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann, C., von Mollard, G.F., Jensen, O.N., Margolis, N., Stevens, T.H., and Wickner, W. (1999). Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol. 145, 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater, C.A., Raymond, C.K., Ekena, K., Howald-Stevenson, I., and Stevens, T.H. (1992). The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J. Cell Biol. 119, 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mollard, G.F., Nothwehr, S.F., and Stevens, T.H. (1997). The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J. Cell Biol. 137, 1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., Emr, S.D., and Riezman, H. (1998). Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Whyte, J.R., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637. [DOI] [PubMed] [Google Scholar]
- Wooding, S., and Pelham, H.R. (1998). The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell 9, 2667–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]