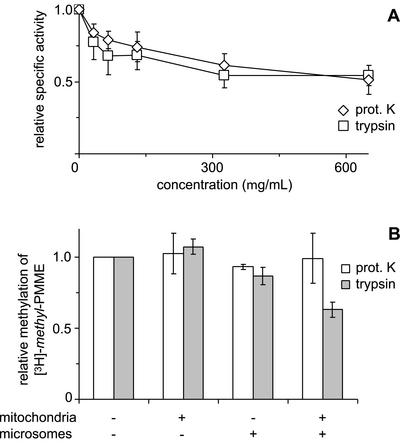
Figure 5.
Effects of treatment with proteases on (A) the enzyme activity of Opi3p and (B) the methylation of mitochondrial [3H]-methyl-PMME in the reconstituted system. (A) Microsomes from a cho2Δ strain were incubated with the indicated concentrations of proteinase K or trypsin before incubation with [methyl-3H]AdoMet to assay enzyme activity. TLC analysis revealed that >90% of the lipid-incorporated label ended up in PC over the entire range of protease concentrations tested. Data have been normalized to the specific activity of Opi3p in mock-treated microsomes (n = 3, ±SD). (B) Mitochondria from the opi3Δ strain and/or wild-type microsomes were treated with proteinase K (500 μg/ml) or trypsin (100 μg/ml), as indicated, before coincubation at 1 mg/ml each in the presence of AdoMet. Methylation was quantified as the increase in the percentage of [3H]-methyl label present in PDME and PC upon 2 h of incubation and normalized to that of the control. Data are mean values from two independent experiments, with the error bars representing the variation.