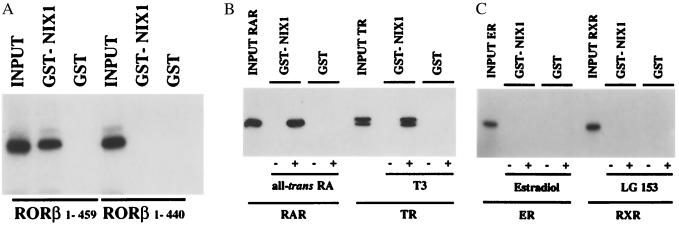
Figure 3.
Selective interaction between NIX1 and nuclear receptors in vitro. GST protein alone or NIX1 fused to GST was immobilized on glutathione-Sepharose beads, and GST-pulldown experiments were performed with in vitro translated [35S]methionine-labeled nuclear receptors and appropriate ligands or vehicles. Autoradiographs show NIX1-interacting nuclear receptors in comparison to 10% of the corresponding input. (A) Full-length RORβ in contrast to the AF2-D deletion mutant RORβ1–440 interacts with NIX1 in vitro. (B) NIX1 associates with RARα and TRβ in a ligand-dependent fashion. For RARα and TRβ, the ligands all-trans-retinoic acid (1 μM) and T3 (0.1 μM), respectively, were used. (C) ERα and RXRα fail to interact with NIX1 in the absence and in the presence of their ligands. For ERα, estradiol was used at 0.1 μM, for RXRα, LG153 was used at 1 μM.