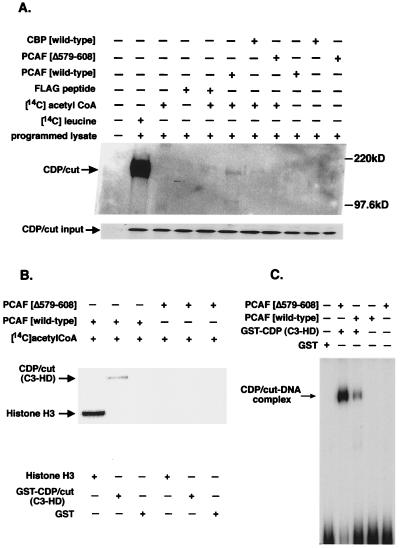
Figure 2.
CDP/cut is a target of acetylation by the HAT PCAF. (A) In vitro acetyltransferase reactions were used on in vitro-synthesized and unlabeled CDP/cut. Equivalent molar amounts of CBP (wild type), PCAF (wild type), and PCAF (Δ579–608) were immunoprecipitated from cells expressing HA-tagged CBP (wild type), FLAG-tagged PCAF (wild type), and FLAG-tagged PCAF (Δ579–608) and used in an in vitro reaction along with [14C]acetyl-CoA where indicated. CDP/cut was synthesized with [14C]leucine in vitro and immunoprecipitated as a control. Immunoprecipitated products were resolved on a SDS/PAGE gel and autoradiographed. Lower corresponds to an immunoblot of the in vitro-translated product of CDP/cut as the input amount for protein acetyltransferase reactions in vitro. (B) Acetylation of C3 and HD of CDP/cut. GST fusion protein of CDP/cut (C3-HD) was affinity purified and subjected to acetylation reactions with the same FLAG-tagged reagents described above. Acetylated product of the GST fusion with CDP/cut was separated by SDS/PAGE and autoradiographed. Free histone H3 served as a positive control for the acetyltransferase activity of PCAF. Gels were stained with Coomassie stain for estimation of protein levels before autoradiography (not shown). (C) Acetylation of CDP/cut disrupts DNA binding to the TK promoter. EMSA of the acetylated product of GST-CDP/cut (C3-HD) after in vitro acetyltransferase reactions as described above with the exception that reactions were performed with unlabeled acetyl-CoA.