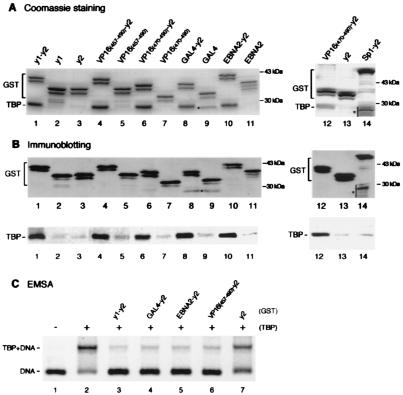
Figure 1.
Acidic activation domains form stable complexes with TBP and inhibit TBP binding to the TATA box when fused to TAND-2. (A and B) VP16 (residues 457–490 or 470–490; lanes 4, 6, and 12), GAL4 (lane 8), EBNA2 (lane 10), and Sp1 (lane 14) activation domains as well as yTAND-1 (lanes 1) were fused to yTAND-2 (y2) and expressed in E. coli as GST-tagged proteins. These activation domains (lanes 5, 7, 9, and 11), yTAND-1 (lane 2), and yTAND-2 (lanes 3 and 13) also were expressed as GST-tagged proteins. GST fusion proteins were incubated with an equimolar amount of yTBP and purified by glutathione Sepharose. The purified materials were analyzed by SDS-PAGE followed by Coomassie blue staining (A) or immunoblotting with anti-GST and anti-TBP antibodies (B). The positions of GST fusions and copurified TBP are indicated. GST fusions appeared as multiple bands due to the protease-hypersensitive sites near the C terminus. Although the bands marked by asterisks in lanes 9 and 14 migrated near TBP, these bands derived from GST fusion proteins according to immunoblotting shown in B. (C) Inhibition of TBP binding to the TATA box by GST-activation domain-TAND-2 chimeras. Gel retardation assays were performed with equimolar amounts of TBP and GST-fusion derivatives that were affinity purified. The positions of TBP-DNA complex and free probe were indicated at the left. Much larger amounts of proteins were required to see the inhibitory effects for activation domains alone (data not shown).