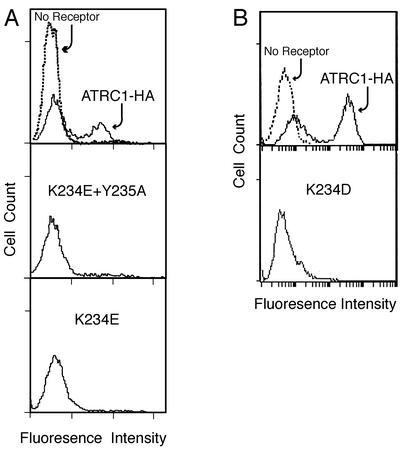
FIG. 3.
A change in lysine 234 correlates with a reduction in virus binding. The markedly reduced binding of the double K234E-plus-Y235A mutant receptor correlates with the decrease in its ability to mediate infection. Single K234E and K234D mutant receptors also produced greatly reduced binding, even though they mediated infection comparably to the wild-type receptor. HEK 293 cells stably expressing the HA-tagged wild-type receptor (mATRC1-HA) or HA-tagged mutant receptors with either combined replacement of lysine 234 by glutamate and tyrosine 235 by alanine (K234E/Y235A) or a single replacement of lysine 234 by glutamate (K234E) or by aspartate (K234D) were incubated with ecotropic Moloney MLV particles at 4°C for 1 h, then with the goat anti-envelope surface protein (SU) at 4°C for 1 h, and finally with mouse anti-goat antiserum conjugated to fluorescein isothiocyanate at 4°C for 1 h. Propidium iodide was added during the final 5 min of incubation with the secondary antibody. Parental HEK 293 cells lacking the ecotropic MLV receptor were treated in the same manner as a negative control (no receptor, dashed line). Relative virus binding to live cells (propidium iodide negative) was quantified by flow cytometry.