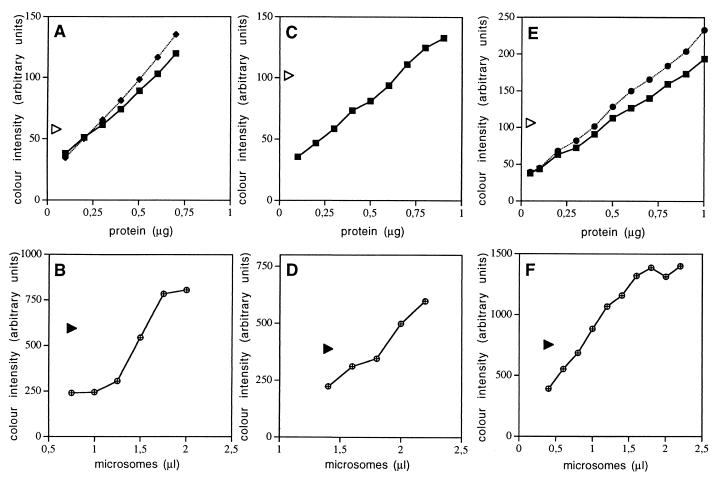
Figure 3.
Quantitation of canine Sec62p and Sec63p in pancreatic microsomes. Serial dilutions of BSA (squares) and carbonic anhydrase (diamonds and circles, respectively) were run on SDS/PAGE gels in parallel to preparations, containing immunoaffinity-purified Sec62p (A), Sec63p (C), and purified Sec61 complex (E), respectively. The proteins were stained with Coomassie brilliant blue and the staining intensity was quantified by densitometry (Personal Densitometer, Applied Biosystems). The same preparations of these purified proteins were run on SDS/PAGE gels in parallel to serial dilutions of dog pancreas microsomes (B, D, and F). Subsequently, the proteins were transferred to PVDF membranes and incubated with specific rabbit antibodies and with a peroxidase conjugate of anti-rabbit IgG-goat antibodies. The bound antibodies were made visible by incubation with enhanced chemiluminescence and exposure to x-ray film. The intensity of silver precipitation was quantified by densitometry. The calculation of the molar concentrations of the various Sec proteins in microsomal suspensions was based on the predicted molecular masses of human Sec62p (45.9 kDa), human Sec63p (88 kDa), and canine Sec61αp (52.2 kDa), respectively, as calculated by the protean option of the dnastar sequence analysis software. The triangles represent the color intensities that were obtained for the purified proteins of interest. We note that the values observed for the purified proteins were within the linear ranges of the titrations.