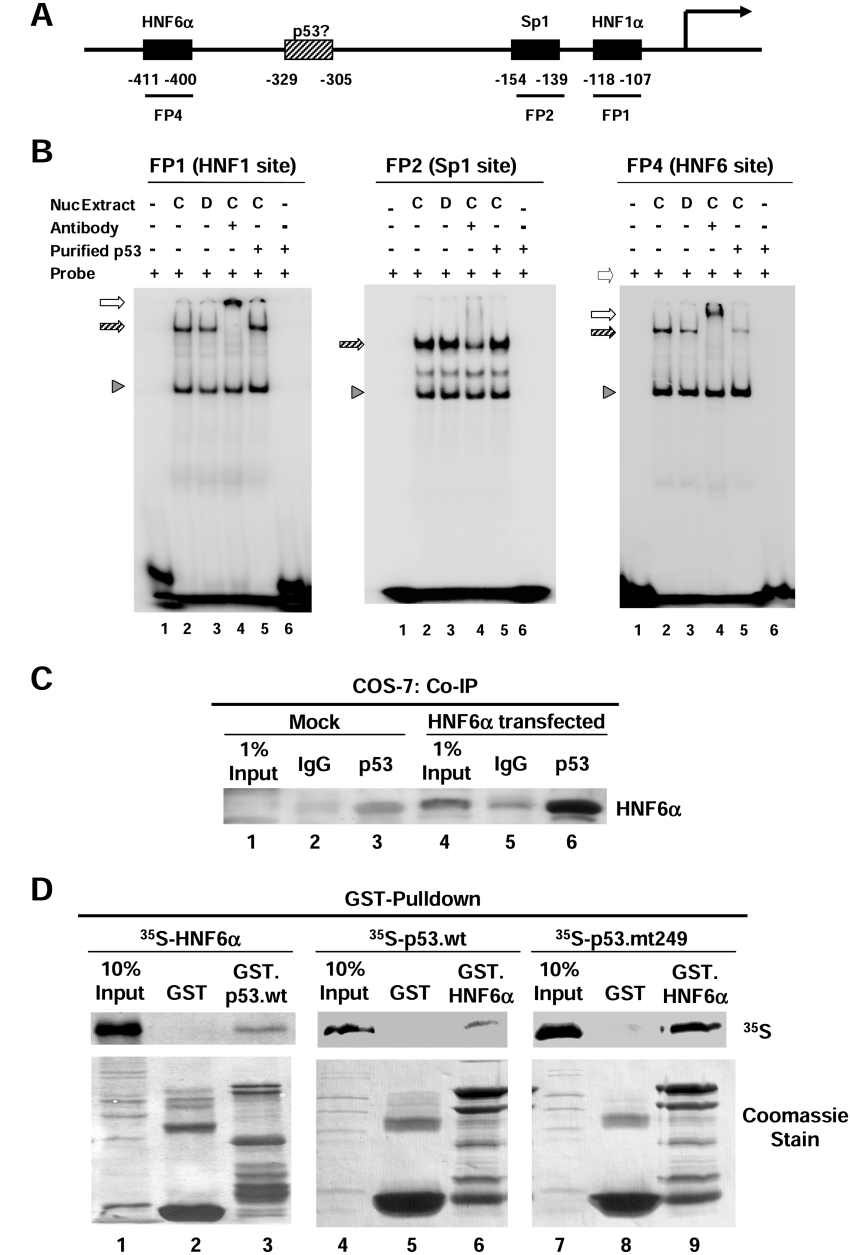
Figure 5. p53 interacts with HNF6α in vivo and in vitro and inhibits its binding to the HNF4α P1 promoter in vitro.
(A) Schematic diagram of HNF4α P1 promoter adapted from [20]. (B) DNA binding activities analysed by EMSA performed with HepG2 nuclear extracts (700 ng each) from control cells (C) or cells treated with doxorubicin (D; 0.5 μg/ml) for 15 h in the presence or absence (+ or −) of purified wild-type p53 protein and 32P-labelled probes of HNF1α, Sp1 and HNF6α response elements of the HNF4α P1 promoter as indicated (FP1, FP2 and FP4). Shift complexes (hatched arrows) were verified by supershift (open arrows) using antibodies against HNF1α or HNF6α for the FP1 and FP4 probes respectively. A non-specific complex (arrowhead) serves as a control for loading and provides evidence of the specificity of the inhibition of HNF6α binding by p53 (right-hand panel, lane 5). Shown are autoradiograms of the shift gels. (C) Co-IP assay using untransfected (Mock) and HNF6α-transfected COS-7 cells. The immunoprecipitation was performed using the mouse monoclonal antibody that recognizes p53 (DO-1; lanes 3 and 6) and mouse IgG as a control (lanes 2 and 5). The immunoblot was performed using anti-HNF6 N-terminus antibody and ECL® detection. Faint bands in the Mock Co-IP (lanes 2 and 3) were most likely caused by cross-reaction with the heavy chain of the antibodies for immunoprecipitation. (D) GST pull-down assay was performed with in vitro-synthesized 35S-labelled HNF6α, 35S-labelled p53.wt or p53.mt249 and immobilized GST, GST.p53.wt or GST.HNF6α proteins as indicated. The autoradiogram of the SDS-gel transferred to Immobilon is shown. Coomassie stain shows the amount of GST proteins loaded in each lane.