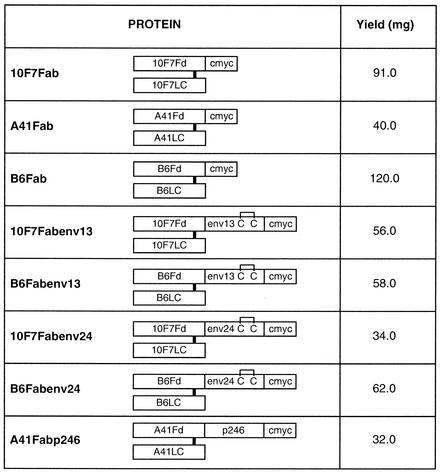
FIG. 1.
Diagrammatic representation of various fusion proteins. Recombinant Fd of an MAb or its fusion protein makes an interchain disulfide bond with the corresponding light chain. These fusion proteins are obtained by assembling the light chain with Fd-Fd fusions containing HIV-derived peptide and by using a protocol of in vitro denaturation of inclusion bodies and subsequent renaturation under proper redox conditions to assemble the functional Fab. The monomeric Fab fusion proteins containing HIV-derived peptides are purified to a high degree and free of aggregates by multistep column chromatographic procedures. The intrachain disulfide bonds of the light chain and Fd are not shown. Fd, antibody fragment containing the variable domain and the first constant domain of the heavy chain; LC, light chain of antibody; env13, 31 amino acids (590 to 620) of HIV-1 envelope gp41 (AVERYLKDQQLLGIWGCSGKLICTTAVPWNA); env24, 31 amino acids (581 to 611) of HIV-2 envelope gp36 (AIEKYLQDQARLNSWGCAFRQVCHTTVPWVN); c-myc, decapeptide recognized by MAb 9E10; p246, amino acids 8 to 153 of HIV-1 capsid protein p24; C, cysteine. The yield corresponds to the amount of Fab obtained from 8 liters of renatured material containing 800 mg of total protein.