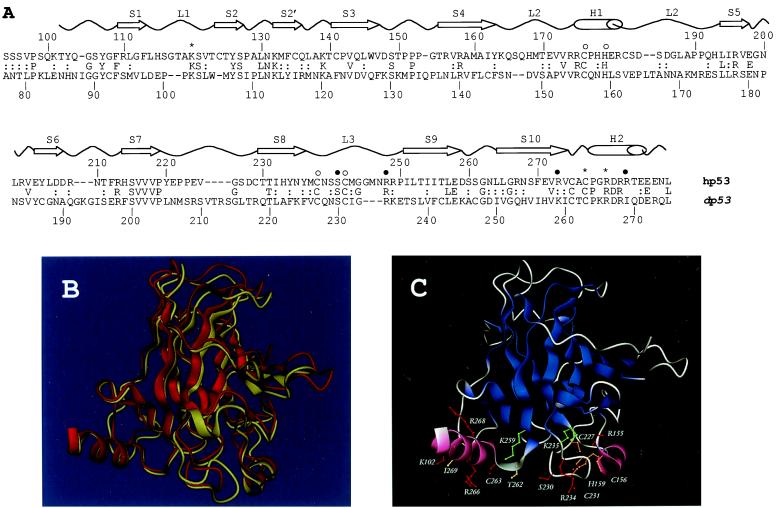
Figure 1.
(A) Sequence and structure comparison of the Drosophila and human p53 DNA-binding domains. Sequence alignment of the dp53 and hp53 DNA-binding domains as produced by psi-blast. The secondary structure elements of hp53 are shown above (S, β-strand; L, loop; H, α-helix). Residues involved in DNA binding (*, contacting bases; ●, contacting phosphate backbone) and zinc binding (○) also are indicated (6). (B) Superimposition of the crystal structure of hp53 (yellow cartoon) DNA-binding domain and the model of the dp53 domain (red cartoon) predicted by program modeller. (C) Protein structure model of the dp53 DNA-binding domain. Color scheme: red, residues preserved between the human and Drosophila sequences; green, conservative substitutions; orange, preserved Zn-coordinating residues; and yellow, nonconservative substitutions. B and C were rendered by program dino (http://www.biozentrum.unibas.ch/∼x-ray/dino).