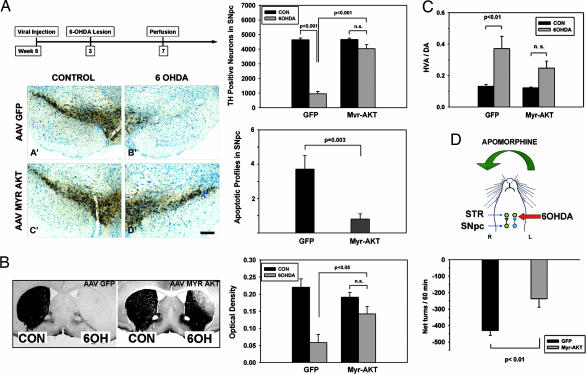
Fig. 3.
Neuroprotective effects of Myr-Akt in the intrastriatal 6OHDA mouse model. (A) (Left Upper) The chronic morphologic studies shown in A and B were performed according to the timeline shown. The studies of apoptosis were conducted at 6 days after 6OHDA. (Left Lower) Mice treated with an AAV GFP control injection show an almost complete loss of TH-positive SNpc neurons. This loss is almost completely prevented in mice injected with AAV Myr-Akt (D′). (Bar: 200 μm.) (Right Upper) This effect is shown quantitatively as stereologic counts of the number of surviving TH-positive neurons. In mice given AAV GFP, TH-positive neuron numbers were reduced to 950 ± 163 after 6OHDA injection, or 20% of the noninjected contralateral control (CON) (4,646 ± 119). In mice given AAV Myr-Akt, neuron numbers were reduced to only 4,036 ± 280, or 87% of the contralateral control (4,664 ± 85). The difference between the AAV GFP and AAV Myr-Akt groups was highly significant (P < 0.001, ANOVA; n = 6 animals GFP; n = 7 animals Myr-Akt). (Right Lower) This protective effect of Myr-Akt on SNpc DA neurons was attributable to suppression of apoptosis as shown. The number of apoptotic profiles in the SNpc was reduced to 22% of the number observed in AAV GFP-treated mice (P = 0.003, t test; n = 7 animals AAV GFP; n = 8 animals AAV Myr-Akt). (B) The neuroprotective effect of Myr-Akt was observed at the level of the striatal axonal projections as well. (Left) In control AAV GFP-injected mice, there is an almost complete loss of striatal TH-positive staining on the 6OHDA-injected side. In AAV Myr-Akt-injected mice, there is some loss of immunoperoxidase labeling in the dorsolateral quadrant of the injected striatum, but otherwise staining of TH fibers is preserved. The mice used for this analysis were the same as those used for the stereologic analysis in A. (Right) The protective effect of Myr-Akt on striatal TH-positive fibers is shown quantitatively as optical densities of TH staining. (C) The morphologic preservation of striatal dopaminergic fibers was accompanied by relative preservation of biochemical indices of dopaminergic terminals. At 4 weeks after 6OHDA, striatal DA levels in AAV Myr-Akt-treated mice were 19.8 ± 4.2 ng per sample, a 3.4-fold increase over the mean value of 5.8 ± 2.9 observed in AAV GFP-treated mice (P < 0.03; n = 6 animals AAV GFP; n = 8 animals AAV Myr-Akt) (data not shown). HVA levels were increased by 3-fold (data not shown). After lesions of the nigrostriatal dopaminergic projection, there is a compensatory increase in DA turnover, reflected in an increased HVA/DA ratio (16). This effect was observed in AAV GFP-treated mice as an increase in the HVA/DA ratio from 0.13 ± 0.01 in the nonlesioned striatum to 0.37 ± 0.08 in the lesioned striatum (P = 0.006). However, in the AAV Myr-Akt-treated mice, although there was a trend for this effect, it did not achieve significance. (D) To assess functionality of the nigrostriatal projection we examined apomorphine-induced rotations. After partial destruction of the dopaminergic projection, postsynaptic supersensitivity to direct-acting DA agonists, such as apomorphine, results in contraversive rotations (29). Strong contraversive rotational behavior was observed in AAV GFP-treated mice after apomorphine injection (0.5 mg/kg) at 4 weeks after 6OHDA lesion. This contraversive rotational behavior was significantly diminished in mice treated with AAV Myr-Akt (AAV GFP, −431 ± 29 net turns per 60 min; AAV Myr-Akt, −237 ± 52; P = 0.006, t test; n = 7 animals GFP; n = 6 animals Myr-Akt).