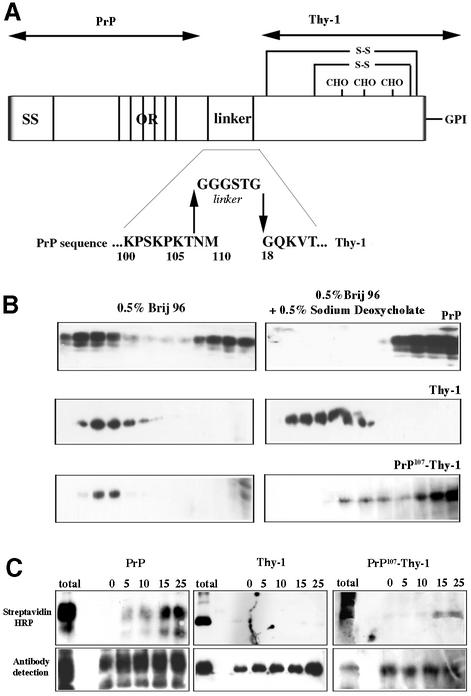
Fig. 7. The N-terminal domain of PrPC is sufficient to specify the solubility and endocytosis of a GPI-anchored protein. (A) Scheme showing the fusion protein PrP107–Thy-1, in which the N-terminal domain of PrPC is joined via a flexible linker to full-length Thy-1. SS, signal sequence of PrP; OR, octapeptide repeats; CHO, the N-linked carbohydrate chains of Thy-1; S-S, disulfide bonds of Thy-1. Amino acids are abbreviated in single letter code. (B) Western blots of density gradient fractions after solubilization of N2a cells in Brij 96 with (right) or without (left) 0.5% sodium deoxycholate, probed for PrPC, Thy-1 or fusion PrP107–Thy-1. (C) Endocytosis measured by protection of biotinylated protein from extracellular reductive cleavage. N2a cells were surface biotinylated, and either solubilized immediately (total) or incubated at 37°C for the number of minutes indicated before external biotin was removed by glutathione cleavage; endogenous PrPC (left) and Thy-1 (middle) or fusion PrP107–Thy-1 (right) were immunoprecipitated and detected using streptavidin–HRP (upper panel) or western blotting (lower panel).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.