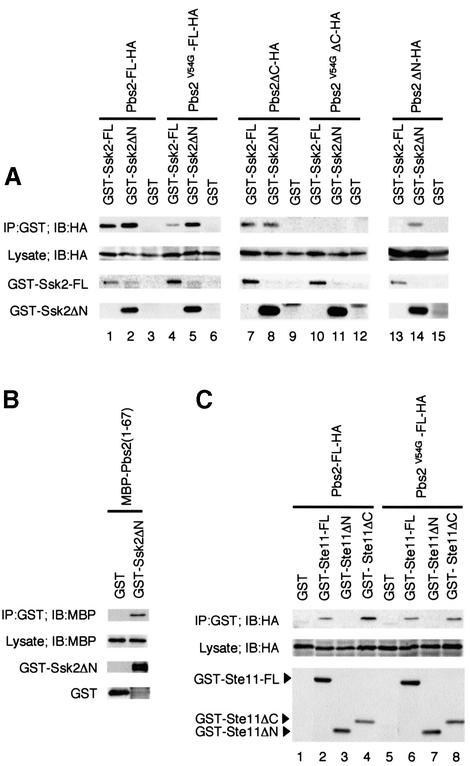
Fig. 4. (A) In vivo binding of Pbs2 to Ssk2. TM261 (pbs2Δ) was co-transformed with a plasmid expressing either full-length GST–Ssk2 (GST–Ssk2-FL), GST–Ssk2ΔN or GST alone, together with a second plasmid expressing either full-length HA-tagged Pbs2 (Pbs2-FL-HA) or its mutant or deleted derivatives, under the PGAL1 promoter. GST fusion proteins were precipitated from cell lysates using glutathione–Sepharose beads as described in Materials and methods. Co-precipitated Pbs2-HA (or its derivatives) was detected by immunoblotting using an anti-HA antibody as shown in the top row. The second row indicates the expression of Pbs2-HA in the total extract. The bottom two rows show the levels of GST–Ssk2-FL and GST–Ssk2ΔN, respectively, in the precipitates (GST alone was expressed at even higher levels; data not shown). (B) Direct binding of Pbs2 RSD-I to the Ssk2 kinase domain. The cell lysate from E.coli expressing MBP–Pbs2(1–67) fusion protein was incubated with purified GST–Ssk2ΔN, or GST, bound to glutathione–Sepharose beads. After incubation, beads were washed and subjected to immunoblot analysis with anti-MBP and anti-GST antibodies. (C) In vivo binding of Pbs2 to Ste11. TM261 (pbs2Δ) was co-transformed with a plasmid expressing either full-length GST–Ste11 (GST–Ste11-FL), GST–Ste11ΔN, GST–Ste11ΔC or GST alone, together with a second plasmid expressing either Pbs2-FL-HA or Pbs2V54G-FL-HA under the PGAL1 promoter. Precipitation of GST fusion proteins from cell lysates and detection of co-precipitated Pbs2-HA (or its mutant form) were performed as described in (A).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.