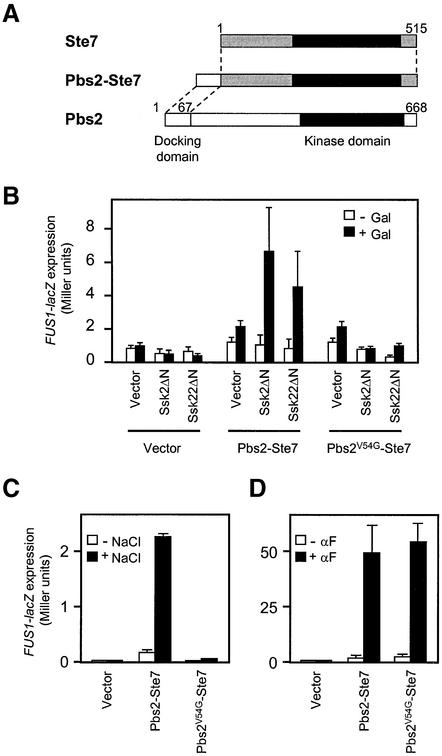
Fig. 5. ‘Cross-talk’ activation of the mating pathway by Ssk2/Ssk22 via binding to a Pbs2–Ste7 fusion protein. (A) Schematic diagram of the Pbs2–Ste7 fusion protein used in this analysis. (B) Induction of FUS1-lacZ expression following activation of the Pbs2–Ste7 fusion protein by constitutively active Ssk2ΔN or Ssk22ΔN. The reporter strain KT007 (pbs2Δ FUS1::lacZ::LEU2) was co-transformed with either pYES2 (Vector), pYES2-Ssk2ΔN or pYES2-Ssk22ΔN together with either YCplac22′ (Vector), YCplac22′-Pbs2–Ste7 or YCplac22′-Pbs2V54G–Ste7. The cells were grown in SRaf medium, and were either harvested (–Gal) or incubated further for 2 h in the presence of 2.5% galactose (+Gal). FUS1-lacZ expression was measured by assaying the β-galactosidase activities in cell lysates as described in Materials and methods. For each combination of plasmids, three independent transformants were assayed in triplicate, and the average activity ± SD is shown. (C) FUS1-lacZ expression induced by osmotic stress via the Pbs2–Ste7 fusion protein. KT005 (pbs2Δ ste11Δ) was co-transformed with a FUS1-lacZ reporter plasmid, pSB231, and either YCplac22′ (Vector), YCplac22′-Pbs2–Ste7 or YCplac22′-Pbs2V54G–Ste7. The cells were grown in CAD and either harvested (–NaCl), or incubated further for 4 h following the addition of 0.4 M NaCl (+NaCl). (D) Restoration of pheromone-induced FUS1-lacZ expression in the ste7Δ mutant by expression of the Pbs2–Ste7 or Pbs2V54G–Ste7 hybrid protein. FP56 (ste7Δ) was co-transformed with pSB231, and either YCplac22′ (Vector), YCplac22′-Pbs2–Ste7 or YCplac22′-Pbs2V54G–Ste7. The cells were grown in CAD and either harvested (–αF), or incubated further for 2 h following the addition of 5 µM α-factor (+αF). FUS1-lacZ expression was measured as in (B).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.