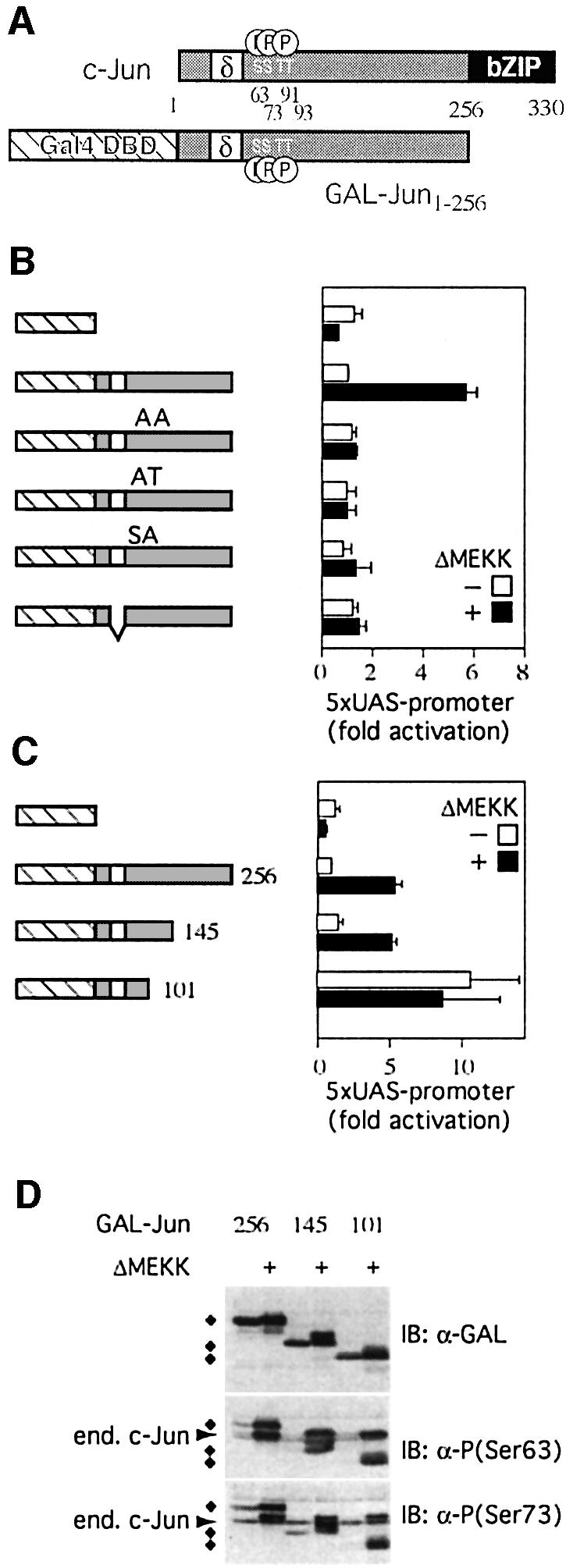
Fig. 1. Features of c-Jun that confer JNK responsiveness. (A) Schematic structure of c-Jun and GAL–Jun: the DNA-binding and dimerization domains of c-Jun (bZIP, black box) and GAL–Jun (GAL-DBD, cross-hatched box), the δ-domain (white box) and the JNK phosphorylation sites (serines 63, 73 and threonines 91, 93) are shown. Numbers indicate amino acid positions. (B) The transcriptional activity of GAL–Jun1–256 MAPK phosphorylation site mutants (AA: serines 63, 73 and threonines 91, 93 were replaced by alanines; AT: serines 63, 73 were replaced by alanines; SA: threonines 91, 93 were replaced by alanines) or a δ- domain deletion mutant were measured in reporter assays with or without activation of the JNK pathway by ΔMEKK. The graph displays the fold increase of normalized reporter activity after transfection with plasmids coding for the indicated fusion proteins relative to GAL– Jun1–256 which was set to 1 (means ± SD of 3–5 independent experiments). (C) Activities of GAL–Jun1–256 and different deletion mutants were determined as in (B). Note that Gal–Jun1–101 is constitutively active. (D) 293 cells were transiently transfected with the indicated expression constructs together with an empty expression vector or an expression plasmid encoding ΔMEKK. Lysates of transfected cells were analyzed by immunoblotting (IB) using either antibodies specific for the GAL-DBD or phospho-c-Jun-specific antibodies against the phosphorylated serine 63 or serine 73 (diamonds indicate fusion proteins, cross-reactivity with endogenous c-Jun is indicated by an arrow).
