Abstract
To induce dissociation of the transcription elongation complex, a typical intrinsic terminator forms a G·C-rich hairpin structure upstream from a U-rich run of approximately eight nucleotides that define the transcript 3′ end. Here, we have adapted the nucleotide analog interference mapping (NAIM) approach to identify the critical RNA atoms and functional groups of an intrinsic terminator during transcription with T7 RNA polymerase. The results show that discrete components within the lower half of the hairpin stem form transient termination-specific contacts with the RNA polymerase. Moreover, disruption of interactions with backbone components of the transcript region hybridized to the DNA template favors termination. Importantly, comparative NAIM of termination events occurring at consecutive positions revealed overlapping but distinct sets of functionally important residues. Altogether, the data identify a collection of RNA terminator components, interactions and spacing constraints that govern efficient transcript release. The results also suggest specific architectural rearrangements of the transcription complex that may participate in allosteric control of intrinsic transcription termination.
Keywords: hairpin/interaction/NAIM/RNA polymerase/termination
Introduction
The formation of a G·C-rich hairpin structure in the nascent transcript upstream from a 3′-terminal U-rich run of 6–8 nucleotides is the hallmark of a widespread class of intrinsic signals for transcription termination [referred to as rho-independent and class I terminators for bacterial and bacteriophage RNA polymerases (RNAPs), respectively; Macdonald et al., 1994; Mooney et al., 1998]. The hairpin fold and the U-tract are believed to represent the minimal core of the terminators (d’Aubenton Carafa et al., 1990), even though other elements such as upstream and downstream DNA template sequences may also affect the efficiency of transcript release (Macdonald et al., 1993; Mooney et al., 1998 and references therein). Despite this apparent simplicity in the morphology of intrinsic terminators, the molecular mechanisms that lead to transcript release at a termination point are not fully understood.
Structural and functional studies on eukaryotic, bacterial and phage RNAPs suggest that similar sets of cooperative interactions between the RNAP, the DNA template and the RNA transcript sustain the high stability of the corresponding transcription elongation complexes (TECs) (reviewed in Landick, 2001; Murakami and Darst, 2003). The completion of these sets of stabilizing interactions is expected once the transition from transcription initiation to processive elongation has occurred, i.e. when a transcript of 10–15 nucleotides has been synthesized (Mooney et al., 1998; Temiakov et al., 2002 and references therein). At this point, the build up of the ∼8 bp RNA–DNA hybrid within the transcription bubble is achieved (Landick, 2001; Tahirov et al., 2002; Yin and Steitz, 2002; Murakami and Darst, 2003) and the 5′ end of the transcript may also associate with a putative RNA-binding site (RBS) within the RNA exit channel of the RNAP (Wilson et al., 1999; Temiakov et al., 2002 and references therein). Induced-fit rearrangements of RNAP substructures upon accommodation of the RNA–DNA hybrid within the transcription complex and upon association of the exiting RNA with the RBS have been proposed to lock the TEC into its highly processive conformation (reviewed in Landick, 2001; Murakami and Darst, 2003). At a terminator, the competitive disruption of the RBS–transcript interaction upon the formation of the terminator hairpin and/or the directional melting of the U-rich RNA–DNA hybrid upon invasion (or active ‘levering’) by the adjacent hairpin may thus induce reversion to a much less stable TEC conformation (Gusarov and Nudler, 1999, 2001; Wilson et al., 1999; Komissarova et al., 2002). In an alternative model, folding of the terminator hairpin mechanically extracts the transcript from a hyper-translocated ternary complex and thus destroys the network of nucleic acid interactions that contribute to TEC stability (Yarnell and Roberts, 1999). Although these models place emphasis on the thermodynamic stability of the terminator hairpin, the sequence arrangement of the motif appears to be equally important (d’Aubenton Carafa et al., 1990; Jeng et al., 1990; Cheng et al., 1991; Macdonald et al., 1993; Hartvig and Christiansen, 1996). Some of these sequence constraints may ensure timely folding of the terminator hairpin (Hartvig and Christiansen, 1996). However, unrecognized structural features of the terminator hairpins or transient, termination-specific, tertiary contacts with the other TEC constituents may also be salient components of the termination signals.
The investigation of the mechanisms of termination is complicated by the dynamic nature of transcription. For instance, intrinsic terminators trigger transcript release at several consecutive template positions that define the window of opportunity for termination (McDowell et al., 1994; Wilson and von Hippel, 1994). It is usually assumed that this distribution of termination products arises from kinetic partitioning governed by invariant molecular determinants (McDowell et al., 1994; Wilson and von Hippel, 1994; Boudvillain et al., 2002). Nevertheless, distinct mechanisms for transcript release at consecutive positions cannot be formally excluded. Given these uncertainties, a detailed description of transcription termination that would fully integrate its kinetic and molecular aspects is still lacking. This is particularly true since potential flaws in key experiments devoted to the analysis of intermediate states of transcription termination have been discovered (Kashlev and Komissarova, 2002).
To study the molecular features of transcription termination in its true, in-bulk, dynamic context, we have taken advantage of the nucleotide analog interference mapping (NAIM) strategy. NAIM is a combinatorial approach that provides fast and efficient identification of the atoms and molecular moieties that are functionally important within an RNA molecule (reviewed in Strobel, 1999). NAIM rests on random enzymatic incorporation of NαS analogs into RNA transcripts. The modified transcripts can then be used in any functional assay that permits fractionation of the initial RNA pool into subsets of active and inactive molecules. The molecular modifications (carried by the base, sugar or phosphate moieties of the NαS residues) that interfere with the RNA function are then simultaneously, yet individually, identified by comparison of the NαS incorporation patterns of the RNA subsets after specific cleavage of the phosphorothioate linkages with iodine (Strobel, 1999). By introducing a few modifications to the standard NAIM protocol, we have implemented this chemogenetic approach to the molecular dissection of transcription termination. In this study, the functional anatomy of the T1 terminator of the rrnB gene of Escherichia coli has been investigated during transcription with T7 RNAP, and the RNA atoms and functional groups that participate in the mechanisms of transcript release have been identified. Collectively, the results reveal striking molecular features of the terminator RNA that are critical for both TEC inactivation and destabilization during transcription termination.
Results
A NAIM assay for transcription termination
To identify RNA molecular components involved in transcription termination, we sought to adapt the standard NAIM protocol. In effect, the very nature of transcription termination enables merging of the first NAIM steps (preparation of the RNA pool and transcript selection) into a single ‘one-pot’ reaction. Hence, DNA templates containing the sequence of intrinsic terminators can be transcribed in the presence of NTPαS, and the resulting, randomly modified, transcripts that are released at the termination point and at the template end can be separated by gel electrophoresis. Then, NAIM interference signals can be deduced directly from comparative iodine sequencing of the two transcript populations. In theory, this experimental framework is applicable to every RNAP. However, one limiting condition is the efficient incorporation of NαS analogs into transcripts without dramatic alteration of the fidelity or catalytic features of the RNAP. While these requirements have not been yet evaluated extensively for any bacterial or eukaryotic RNAP, the Y639F mutant of T7 RNAP readily utilizes non-natural rNTPs (such as 2′-modified NTPs; Huang et al., 1997) and is already commonly used in standard NAIM (Boudvillain and Pyle, 1998; Ortoleva-Donnelly et al., 1998a,b). Moreover, key features of transcription termination are similar for the wild-type and Y639F enzymes (Figure 1A; Boudvillain et al., 2002). The Y639F mutant can thus be used advantageously in the NAIM of termination events occurring during transcription with T7 RNAP. Importantly, control experiments with NTPαS analogs that are substrates for both the Y639F and wild-type T7 RNAPs (Ortoleva-Donnelly et al., 1998b) yielded similar NAIM signals for the two enzymes (data not shown).
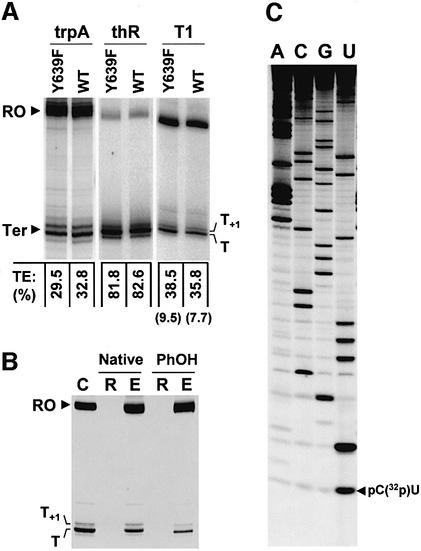
Fig. 1. Transcription termination with wild-type (WT) and mutant (Y639F) T7 RNAPs. (A) The run-off (RO) and termination (Ter) products obtained with linearized plasmids pAST7-T1 (T1), pMBT7-trpA (trpA) and pMBT7-thR (thR) were resolved on 9% denaturing poly acrylamide gels. Global termination efficiencies (TEs) are indicated below the lanes. Individual TEs at the minor release site (T+1) of the T1 terminator are enclosed in parentheses. (B) Fractionation of a transcription reaction with Y639F RNAP (lane C) on 100 kDa Microcon columns before (lanes Native) or after (lanes PhOH) phenol extraction and ethanol precipitation. Lanes R and E refer to the membrane retenate and eluate fractions, respectively. (C) Identification of transcript 3′ ends. Phosphorothioate sequencing was performed on 3′-end-labeled T transcripts as described previously (Boudvillain et al., 2002). Minor T+1 transcripts with labeled 3′ ends could not be prepared in sufficient amount for direct comparison.
Analysis of RNA release at the rrnB T1 terminator
The rrnB T1 terminator triggers RNA release from the T7 TEC by a mechanism that depends on hairpin formation 6–8 nucleotides upstream from the transcript 3′ tip (class I termination; Boudvillain et al., 2002 and references therein). Interestingly, the terminator hairpin is capped by a GNRA loop, a common RNA motif whose structure (Hermann and Patel, 1999), thermodynamics (SantaLucia et al., 1992; Rife et al., 1998) and NAIM signature (Boudvillain and Pyle, 1998) have been characterized in various contexts and thus could be useful in interpreting the results. However, a mechanistically distinct termination signal (class II signal) is also imbedded in the T1 sequence (Hartvig and Christiansen, 1996; Lyakhov et al., 1997), which could obscure the analysis of the upstream terminator. To study the latter in isolation, the class II template sequence 5′-ATCTGTT (Lyakhov et al., 1997) has been mutated to 5′-ATCTATC. This modification selectively abolished class II termination (Figure 1A; He et al., 1998). The mutated T1 DNA template (plasmid pAST7-T1 linearized with EcoRV) was thus transcribed with the Y639F RNAP in the presence of one of the NTPαS probes (see Materials and methods for the names and abbreviations of the analogs). To ensure the selective recovery of transcripts released from ternary complexes, transcription mixtures were filtered through size-exclusion membranes under conditions that prevent non-specific rebinding of RNAP to the transcripts (Figure 1B; data not shown; see Materials and methods) and that eliminate high molecular weight species such as arrested ternary complexes (Gopal et al., 1999). Transcripts were then radiolabeled and purified by polyacrylamide gel electrophoresis under strong denaturing conditions (see Materials and methods). After iodine treatment of the purified transcript subsets, the positions of NαS incorporations that interfere with T1 transcription termination were identified. In a first set of experiments, only the transcripts released at the strongest termination site (T site in Figure 1A) were examined. Using a discrimination factor (λ) and a statistical threshold for NAIM effects (see Materials and methods and Figure 2B), only 12% of the NαS incorporations were found to interfere significantly with termination. Moreover, most of the interference effects are located in the terminator hairpin and the U-rich downstream portion of the transcript (Figures 2 and 3A), the two RNA components of the termination signal (Jeng et al., 1990; Mooney et al., 1998). This particular distribution of effects is a good indication that the NAIM assay monitors termination appropriately and thus provides a convincing argument in favor of the approach.
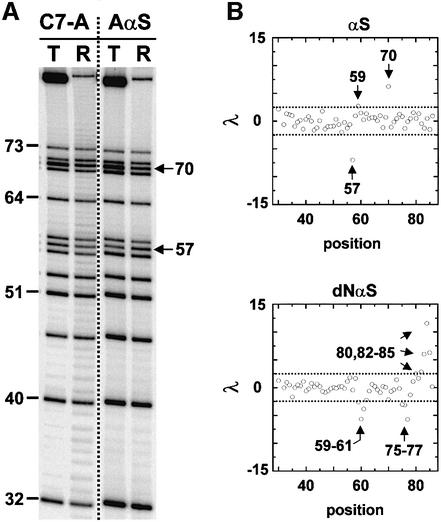
Fig. 2. NAIM of the transcripts released at the major site (T) of the T1 terminator. Numbering of the nucleotide positions is from the +1 start site of transcription. (A) Iodine sequencing of termination (T) and run-off (R) products of transcription reactions containing either ATPαS (AαS) or 7-deaza-ATPαS (C7-A). The positions of NAIM effects are indicated by arrows. (B) The identification of interference effects (arrows) using the discrimination factor λ (see Materials and methods) is presented for the Rp-phosphorothioate (αS) and 2′-deoxy (dNαS) incorporations. Dotted lines correspond to the cut-offs (±2.5) that were applied to select statistically significant NAIM effects.
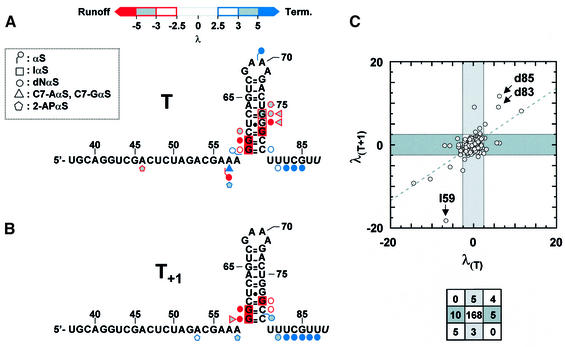
Fig. 3. Summary of the NαS effects on termination at the (A) T site; (B) T+1 site. Only the NαS analogs that yielded interference signals are shown (key is inset). The positions of NαS effects are indicated on the secondary structure of the transcripts (upstream transcript positions did not exhibit NαS effects and are not shown). The levels of NαS incorporations could not be quantified accurately for the last 3′-nucleotide of the transcripts (in italics). The key to color coding of weak, moderate and strong effects is shown above the sequence of the T transcript. (C) Comparison of NαS effects on termination at the T and T+1 sites using the discrimination factor λ (see Materials and methods). The dashed line is the best linear fit of the data points (y intercept = 0.07; slope = 0.67). Dark and light gray regions contain NαS modifications selectively affecting termination at the T and T+1 sites, respectively. Arrows identify IαS and dNαS effects at positions 59, 83 and 85. The number of hits per graph region is depicted schematically below the diagram.
Only three single-point phosphorothioate (αS) modifications of the transcripts (5′ of A57, G59 and A70) altered termination (Figures 2 and 3A). In addition, the three αS effects were not strong enough to preclude further NαS mapping of the corresponding positions (provided that NαS signals are corrected for αS effects; see Materials and methods). Phosphorothioate effects may reflect diverse structural or electronic perturbations (Boudvillain and Pyle, 1998 and references therein). However, only the loss of critical metal–phosphate interactions upon αS substitution can be probed easily in rescue experiments with thiophilic metal ions such as Mn2+ or Cd2+ (examples can be found in Ortoleva-Donnelly et al., 1998b). Unfortunately, addition of thiophilic ions to the reaction mixtures altered transcription features in various ways (Boudvillain et al., 2002; M.Boudvillain, unpublished results), thereby preventing reliable rescue experiments to be performed. Still, the termination-enhancing αS effect within the GAAA loop (λ = 6.2; Figure 2B) is intriguing because the corresponding pro-Rp oxygen (5′ of A70) is not involved in contacts within the GNRA fold (Hermann and Patel, 1999 and references therein). In addition, this is the only NAIM effect that has been observed within the tetraloop (Figure 3A). Neither the many NαS incorporations (IαS and dGαS at G68; C7-AαS, RαS, and 2-APαS at A70–71; αS at A71) that should have disrupted GAAA intramolecular H-bonds (Hermann and Patel, 1999 and references therein) nor those altering functional groups lying on the exterior of the structure (αS at G68 and A69; dNαS at G68 and A69–71; C7-GαS at G68; C7-AαS, RαS and 2-APαS at A69) yielded interference signals (Figure 3A). These data suggest that isolated functional groups of the tetraloop are not primary determinants of the termination event nor is the integrity of the GAAA motif essential to hairpin stability in this context. On the other hand, the distinct properties of an Rp-S atom on the 5′ side of A70 may improve other features of the terminator capping loop (such as timely folding).
With a few exceptions (C7-AαS at A57; 2-APαS at A46 and A57), all the modifications of the nucleotide bases that perturbed transcription termination lie within the lower half of the hairpin stem (Figure 3A). In particular, IαS effects are likely to reflect the necessity for strong base pairing in this region during termination. This proposal is supported by the absence of mGTPαS interference effects within the terminator hairpin (Figure 3A; data not shown). While N2-methylguanosine (mG) and guanosine form iso-energetic pairs with C residues, the methyl group prevents tertiary contacts to the N2 moiety of an mG·C pair (Ortoleva-Donnelly et al., 1998a). The absence of mGαS effects therefore rules out functionally important tertiary contacts to the N2 exocyclic amines of the hairpin G·C pairs whereas it assigns unequivocally the termination-reducing IαS effects at G59, G60, G76 and G78 (Figure 3A) to thermodynamic destabilization of the terminator hairpin (one H-bond is destroyed in each case). Similarly, the IαS and C7-GαS effects at G77 (Figure 3A) could reflect the disruption of a stabilizing hydration pattern that is sometimes observed for G.U pairs and that has been implicated in RNA recognition by proteins (Mueller et al., 1999). This suggests that termination-specific contacts could occur between the RNAP and the hairpin G77·U62 pair. The particular structural features of G·U pairs could also favor direct RNAP contacts to the N7 and/or N2 moieties of G77 (without water mediation; Hermann and Patel, 1999) and allow access to additional interacting components in the major groove of the adjacent G76–C63 pair (as suggested by the C7-GαS interference at G76; Figure 3A).
Another set of significant effects on transcription termination corresponds to the multiple dNαS interference signals located in the hairpin stem and U-rich transcript 3′ end (Figures 2B and 3C). Interference effects due to single deoxy substitutions within an RNA secondary structure (such as RNA hairpins) are usually taken as evidence for disruption of important interactions involving the corresponding 2′-OH moieties (Boudvillain and Pyle, 1998; Ortoleva-Donnelly et al., 1998b; Szewczak et al., 1998; Strobel, 1999; Boudvillain et al., 2000). The same probably holds true for RNA–DNA hybrids, as single deoxy substitutions should not strongly affect hybrid structure or stability (Freier and Altmann, 1997; Sugimoto et al., 2000 and references therein). Thus, the dNαS effects observed for the termination species (Figures 2B and 3A) most probably reflect the involvement of the corresponding 2′-OH groups in contacts with the RNAP that have to be formed (λ < –2.5) or broken (λ > 2.5) for successful termination. In the latter case (dNαS effects with λ > 2.5), additional contributing factors may include subtle effects on RNAP catalysis (slowing down transcript extension) or on the conformation of the RNA–DNA hybrid within the TEC (indirectly weakening RNAP contacts to the RNA–DNA hybrid; see below and Tahirov et al., 2002).
Different RNA functional components are involved in transcription termination at the T and T+1 sites
Another transcript subset that is directly related to the T1 termination event corresponds to the RNA species that are released at the minor T+1 site (Figure 1A and B). Thus, the T+1 products randomly modified with NMPαS were also collected and their NAIM patterns compared with those of run-off transcripts. Significant differences in the nature, position or intensity of the interference effects were observed compared with the major T products (Figures 3 and 4). Hence, of the 32 individual RNA modifications that affect transcription termination, only nine are common to the T and T+1 events (Figure 3C). Importantly, including the T+1 signals in the normalization of run-off signals did not affect the results for the T products (not shown). This is due to the low amount of the T+1 species that is formed in comparison with the amount of run-off transcripts (<10%; Figure 1A) and, to a lesser extent, to the different positions and generally weaker intensities of the interference effects on T+1 termination (Figure 3C).
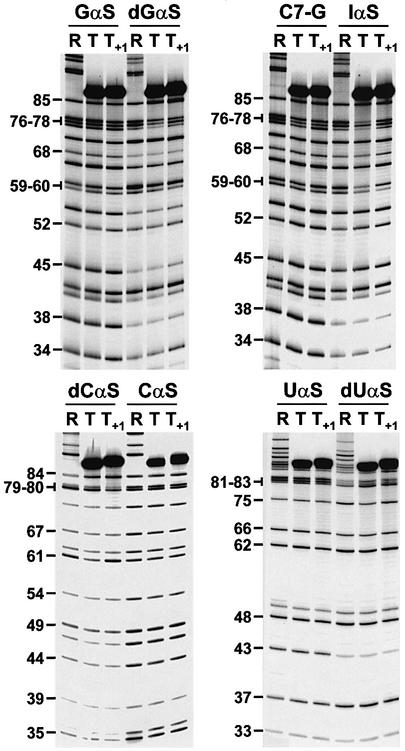
Fig. 4. Comparative NAIM of the major (T) and minor (T+1) T1 termination products. Representative sequencing gels showing Rp- phosphorothioate (NαS), 2′-deoxy (dNαS), 7-deaza-GαS (C7-G) and inosine-αS (IαS) modification effects. Lanes R refer to the run-off transcripts.
Despite the many differences between the NAIM patterns of the T and T+1 transcripts, none of the NαS incorporations induced opposite effects on the two termination events (Figure 3C). Moreover, the NαS effects on T+1 termination are also located mainly within the lower half of the hairpin stem and the transcript 3′ end (Figure 3B). This suggests that the distinct NAIM patterns reflect a change in register within the TEC and, possibly, distinct steps of the same reaction pathway, rather than completely different mechanisms. In this context, comparative NAIM analysis of the two termination events can be summarized as follows. (i) In both cases, termination-reducing NαS effects (λ < –2.5) are found predominantly within the hairpin stem, whereas enhancing effects (λ > 2.5) mostly lie within the U-rich transcript 3′ end. This suggests that for termination to occur, interactions need to be made within and with the hairpin stem (termination-specific interactions such as hairpin G·C pairings and RNAP contacts with discrete hairpin N7 atoms and 2′-OH groups; see above) but disrupted when involving the transcript 3′ end (RNAP stabilizing contacts to the RNA–DNA hybrid within the TEC; Tahirov et al., 2002). Termination-enhancing signals may also be partly explained by subtle effects on RNAP catalysis (see above). (ii) NAIM effects common to T and T+1 termination are likely to reflect crucial steps along the termination pathway. These effects include strong termination-reducing IαS effects at G59, G60 and G78 (which demonstrate the steady requirement for complete hairpin formation during termination), termination-reducing dNαS effects at G59 and G60 (in favor of conserved RNAP contacts to the base of the hairpin stem during termination) and termination-enhancing dNαS effects at U82, U83, C84 and G85 (which suggest that disruption of contacts to the RNA–DNA hybrid contributes significantly to the kinetics of RNA release at the two sites). (iii) At the T+1 site, there is a general shift in the location of termination-reducing effects towards the base of the hairpin stem (compare Figure 3A and B). Thus, distinct sets of RNA–RNAP contacts are made during the two termination events which may be related to a change in topological register imposed by TEC translocation one nucleotide further. Alternatively, the T and T+1 NAIM patterns may represent distinct ‘maturation’ snapshots of the TEC progression along similar termination pathways. (iv) Termination-reducing NAIM effects within the hairpin stem are less abundant for the T+1 signal whereas favorable dNαS effects (λ > 2.5) within the U-rich transcript 3′ end are more abundant and stronger (Figure 3). Overall, termination-reducing effects are dominant for transcript release at the T site (Σλi = –22.71) whereas effects that reduce and enhance T+1 termination are balanced (Σλi = 0.21). These data suggest a termination mechanism governed by at least two consecutive steps. The first one would include the formation of the hairpin structure and establishment of termination-specific contacts within the TEC (associative step). In the second step, transcript release would be limited by disruption of the intricate network of RNA–RNAP interactions, especially at the level of the RNA–DNA hybrid (dissociative step). Thus, termination at the T site would be dominated by the initial associative step (Σλi << 0) whereas during termination at T+1, the dissociative step would become equally important (Σλi ∼0). Alternatively, T+1 termination may be more sensitive to other factors such as subtle effects on RNAP catalysis (see above).
Contacts with the 3′ terminus nucleotide during transcription termination
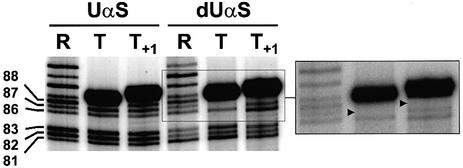
The iodine cleavage of a phosphorothioate linkage occurs on the 5′ side of the NαS residue and yields two RNA fragments, the 3′ fragment being the one that retains the N sugar and base moieties (Gish and Eckstein, 1988). Thus, provided that the 32P label is introduced at the 5′ end of the transcript, iodine cleavage makes NAIM possible up to the very last 3′ residue. This is illustrated in Figure 5 where fragments migrating slightly faster than the full-length species correspond to the cleavage products of transcripts that had UαS residues at their 3′ tip (see also Figure 1C for identification of the transcript 3′ ends). However, even with optimized conditions of gel migration, the very strong signals corresponding to the full-length species did not permit accurate quantification of underneath bands (Figure 5; data not shown). Still, highly inefficient dUαS incorporation at the 3′-most position of the T and T+1 termination species is evident from visual inspection of the sequencing gel (Figure 5). This is in sharp contrast to the multiple strong termination-enhancing dNαS effects observed at positions directly upstream from the transcript 3′ tip (Figure 3) and demonstrates the requirement for a 2′-OH at this position. One possible explanation for this requirement is that termination critically depends on RNAP contacts to the 3′-most 2′-OH group of the transcript. Interestingly, in the T7 TEC crystal structures (Tahirov et al., 2002; Yin and Steitz, 2002), the 2′-OH moiety of the last incorporated ribonucleotide is in close proximity to Asp812 (Figure 6A), an amino acid that is critical for RNAP catalysis (Woody et al., 1996) and that may contribute to TEC inactivation during transcription termination (Boudvillain et al., 2002).
Fig. 5. RNAP contacts to the 3′ tips of transcripts. An enlarged view of a gel showing UαS and dUαS signals at 3′-terminal positions of the T and T+1 transcripts. The dUαS region is also shown with a lower gel exposure. Gel migration has been increased to improve the resolution of the 3′ region (compare with Figure 4). Gel lanes are annotated as in Figure 4. Strong termination-reducing dUαS effects at the transcript 3′ tips are indicated by black triangles.
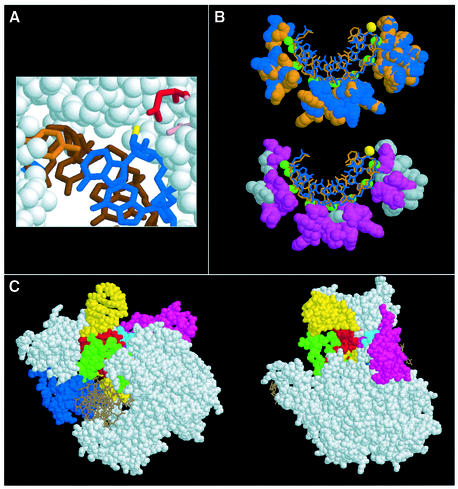
Fig. 6. Steric clashes and interactions between a terminator RNA and important components of T7 RNAP. (A) The transcript 3′ end (in blue), and Y639 (orange), D537 (pink) and D812 (red) RNAP side chains in the active site of a T7 TEC (Yin and Steitz, 2002). The DNA template is in brown and the 2′-OH group of the 3′-ribonucleotide is in yellow. (B) Top: superimposition of the RNA transcript and RNAP interacting residues from two different T7 TEC structures (PDB 1MSW and 1H38 are orange and blue, respectively). The 2′-hydroxyls are green, except for the one of the 3′-ribo nucleotide, which is yellow. Bottom: hydrophobic RNAP residues are shown in gray and polar/charged residues in purple. (C) Docking of a 22 nucleotide hairpin (PDB: 1F9L) similar to the T1 hairpin (same loop but slightly different stem sequence) into the RNA exit channel of the T7 TEC (PDB: 1MSW) using the DeepView program (Guex and Peitsch, 1997). The hairpin 3′ end and the 5′ end of the seventh nucleotide of the TEC transcript have been linked manually. None of the various orientations of the hairpin that have been tried with respect to the TEC were devoid of steric clashes between the RNAP and exiting RNA. Because our primary intent was to provide plausible and general information about the respective positions of terminator and RNAP components and because the T7 TEC is likely to undergo substantial conformational rearrangements upon hairpin formation, we did not attempt further model optimization. The first 5 bp of the terminator hairpin that contain strong NαS effects are red. Other RNA residues are yellow; DNA strands are brown. Major RNAP mobile substructures (Tahirov et al., 2002; Yin and Steitz, 2002) are colored as follows: specificity loop (739–772) in green, flap domain (152–205) in blue, C-linker (258–266) in cyan, N-subdomain (2–71) in magenta. The transcript region upstream from the terminator hairpin is not represented. The figure has been prepared with Protein Explorer 1.98 (http://proteinexplorer.org).
Discussion
The detailed analysis of many individual transcription events is significantly hindered by the complex nature and extensive dynamics of transcript elongation. To circumvent some limitations of classical biochemical methodologies, we have developed a novel experimental approach based on combinatorial NAIM. This approach, which reflects appropriately in-bulk transcription dynamics, allowed the identification of RNA molecular components implicated in intrinsic transcription termination. In this initial study, the NAIM assay has been used to investigate RNA release at the hairpin-dependent T1 terminator during transcription with T7 RNAP. Although parts of this molecular analysis are likely to be specific to the monomeric phage enzyme, many conclusions should also be relevant for transcription termination with multisubunit RNAPs. This is not only because all DNA-dependent RNAPs carry out the basic steps of transcription in an identical manner, but is also due to the many similarities in the structural organization of T7 and multisubunit TECs (Murakami and Darst, 2003). In the forthcoming sections, we utilize the recent T7 TEC structures (Tahirov et al., 2002; Yin and Steitz, 2002) and other previous experimental evidence to interpret our results and discuss the molecular features of intrinsic termination in a way that should also be globally pertinent for multisubunit RNAPs.
Discrete RNA functional groups are important for transcription termination
Formation of the terminator hairpin without significant opening of the RNA exit channel could constitute the driving force for forward movement of the RNAP and transcript extraction from the TEC (hyper-translocation model for termination; Yarnell and Roberts, 1999). Alternatively, RNAP conformational rearrangements resulting from the invasion of the RNA exit channel by a three-strand RNA motif (composed of the terminator hairpin and upstream folded-back section of the transcript) could lead to catalytic impairment and complex destabilization (Gusarov and Nudler, 1999; Wilson et al., 1999; Landick, 2001; Komissarova et al., 2002). In any case, two distinct sets of terminator molecular components seem critical to overcome the energetic barrier to transcription termination. The first set comprises the functional groups that participate in G·C pairings within the lower half of the hairpin stem. Removal of a single H-bond in this region is sufficient to impair termination strongly at both consecutive T and T+1 sites (Figure 3). The second set is composed of discrete hairpin atoms and functional groups involved in termination-specific interactions with the RNAP (see Results and below). The few NAIM effects located upstream from the terminator hairpin (Figure 3) may represent NαS modifications of a folded-back transcript section that either enrich (λ > 2.5) or perturb (λ < –2.5) this set of interactions. Overall, the terminator hairpin sequence is important for thermodynamic stability and correct presentation of specific hairpin molecular components for participation in tertiary interactions with the RNAP. In this perspective, it appears that the particular features of G·U pairs may sometimes be selected to improve and/or enrich these RNAP contacts to the hairpin minor (such as G77-N2) or major (such as G76-N7 and G77-N7) groove components (see Results). Conversely, the permutation of base pairs in the hairpin stem, which impairs transcription termination (Jeng et al., 1990; Cheng et al., 1991), should result in structural changes sufficient to weaken the network of termination-specific interactions. Surprisingly, the hairpin GNRA loop, a structural motif often found in intrinsic terminators (d’Aubenton Carafa et al., 1990), does not participate in these interactions and is not essential to hairpin stability (see Results). The latter observation is consistent with previous work favoring structural over thermodynamic factors to explain the phylogenetic preference for GNRA loops (SantaLucia et al., 1992). Therefore, the terminator GAAA loop may simply promote a U-turn of the RNA chain and favor rapid hairpin folding. Alternatively, the recurrence of tetraloops within terminator hairpins (d’Aubenton Carafa et al., 1990) may be related to functional constraints specific to bacterial RNAPs. As seen in Figure 6C, the above observations are in good agreement with a molecular model of the T7 TEC wherein the GAAA loop of the terminator hairpin (in yellow) lies outside of the structure, whereas the lower half of the hairpin stem (in red) makes extensive contacts (and clashes) with RNAP mobile substructures (in green and magenta) that contribute to TEC stability and processivity. Although this particular representation of the data does not rule out the hyper-translocation model unambiguously, it provides an attractive molecular basis for an allosteric control of termination, which is discussed further below.
Inactivation of transcript elongation at an intrinsic terminator
It has been suggested that a TEC inactivation step (pausing) precedes transcript release at a terminator (reviewed in Mooney et al., 1998; Landick, 2001). Recently, the inactivation of transcript elongation during termination of transcription with T7 RNAP has been linked to subtle variations in the organization of the RNAP active center (Boudvillain et al., 2002). These variations were proposed to disrupt the catalytic coordination network that mediates the two metal ion-assisted addition of nucleotides in the RNAP active site (Steitz, 1998). Two catalytic Mg2+ ions are positioned in the T7 TEC by evolutionarily conserved amino acids D537 and D812 (Woody et al., 1996). In the T7 TEC crystal structures (Tahirov et al., 2002; Yin and Steitz, 2002), an H-bond between the 2′-OH group of the last transcript residue and D812 suggests that this amino acid also participates in the positioning of the transcript 3′ tip within the catalytic coordination network (Figure 6A). Strong termination-reducing dUαS effects at the 3′ ends of termination species (Figure 5) now reveal a functional link between this H-bond interaction and transcription termination (see Results). We propose that this functional link reflects TEC inactivation through a limited alteration of the catalytic coordination network. Whether the alteration results from a displacement imposed on D812 by the interacting 2′-OH group (such as resulting from a particular conformation of the U-rich RNA–DNA hybrid) or from a misalignment of the transcript 3′ tip governed by the 2′-OH–aspartate interaction (allosteric mechanism; see Toulokhonov et al., 2001) awaits further work.
Mechanism of transcript release at a termination site
To investigate the spatial relationship between the RNA hairpin and the terminator boundary, comparative NAIM analysis of transcripts released at consecutive positions has been performed. The results clearly show that, as the RNAP moves further downstream, RNA–RNAP contacts become less abundant and are restricted to the base of the hairpin stem (Figure 3). Moreover, completion of the hairpin stem during termination at the T site should weaken the 8 bp RNA–DNA hybrid by direct invasion of the upstream hybrid base pair (Figure 3A). At the T+1 site, however, hairpin and hybrid base pairs are no longer mutually exclusive (extension of the hybrid stem by A·U pairs is ruled out by the absence of RαS effects at A56–58; Figure 3; data not shown; see also Jeng et al., 1990) and hybrid weakening upon hairpin completion can only result from indirect effects such as spatial restrictions imposed by the RNAP (Komissarova et al., 2002). While melting of the upstream portion of the RNA–DNA hybrid (Komissarova et al., 2002) could not be verified experimentally with the T1 terminator and available NTPαS analogs, the absence of interference upon IαS incorporation at G85 (Figures 3 and 4) indicates that destabilization of the hybrid downstream region is not a primary determinant of transcription termination. In contrast, multiple termination-enhancing dNαS effects within the transcript 3′ ends (Figure 3) suggest that disruption of RNAP interactions with backbone components of the RNA–DNA hybrid (Tahirov et al., 2002; Figure 6B) is a rate-limiting factor during termination. Moreover, the greater number and extent of these dNαS effects during termination at the T+1 site (see Results and Figure 3) could imply a larger energetic penalty for the disruption of the RNAP–hybrid interactions at this position. Overall, predominant release of termination products at the T position stems from a combination of optimal factors: abundant contacts between the terminator hairpin and key RNAP mobile substructures (Figure 6C); weakening of the RNA–DNA hybrid by direct competition between hairpin and hybrid base pairs (see above); and a potentially lower energetic cost for the disruption of RNAP–hybrid interactions. In contrast, suboptimal arrangements of the terminator components at the other positions of the termination window result in less efficient termination. Additional NAIM experiments performed under different transcription conditions may indicate how the RNAP elongation rate modulates these features (McDowell et al., 1994).
Termination-reducing NAIM effects within the terminator hairpin (Figure 3) revealed discrete termination-specific RNA–RNAP contacts. Tentative modeling of the RNA hairpin within the T7 TEC (Figure 6C) suggests that these contacts are made primarily to RNAP substructures whose conformational rearrangements during the transition from transcription initiation to elongation sustain the sharp increase in the stability of the T7 ternary complex (Tahirov et al., 2002; Yin and Steitz, 2002). Steric clashes between the terminator hairpin and the RNAP substructures are also apparent in the model (Figure 6C). It follows that the discrete termination-proficient RNA–RNAP contacts uncovered in this work may embody both attractive (such as H-bonds) and repulsive (such as steric or electrostatic clashes) interactions. Moreover, the hairpin contacts to the RNAP mobile substructures (which could trigger collapse of the RNA exit channel), the shortening of the RNA–DNA hybrid from its upstream edge and the disruption of RNAP–hybrid interactions suggest a termination pathway mimicking, at least in part, the reversal of the transition from transcription initiation to elongation (Tahirov et al., 2002; Yin and Steitz, 2002). Thus, RNA release at intrinsic class I termination sites is likely to result from a major, allosterically controlled, TEC rearrangement (see also Landick, 2001). Nucleotide analog interference suppression (NAIS) experiments (Szewczak et al., 1998; Strobel, 1999; Boudvillain et al., 2000) should provide further molecular support for this mechanism through the identification of energetic couplings between individual RNAP side chains and terminator RNA components.
Materials and methods
Materials
The T7 RNAPs were prepared as described previously (He et al., 1997). Chemicals and enzymes were obtained from Sigma-Aldrich and New England Biolabs, respectively. The NTPαS analogs were purchased from Glen Research, except for mGTPαS, which was kindly provided by Professor Scott Strobel (Yale University).
The pMBT7-T1, pMBT7-trpA and pMBT7-thR plasmids were obtained by fragment shuffling between the XbaI–Acc65I fragment of plasmid pSP73 (Promega) and double-stranded oligonucleotides containing the sequences of the hairpin-dependent T1 terminator, the trpA terminator (from the tryptophan operon of E.coli) or the threonine (thR) attenuator, respectively (Macdonald et al., 1993 and references therein). Using a similar protocol, the first nine nucleotides that follow the T7 promoter in plasmid pMBT7-T1 were replaced by the sequence 5′-GAGAAGAGGAAGAT to yield plasmid pAST7-T1.
Transcription experiments
Transcription mixtures contained 1.1 pmol of plasmid linearized with EcoRV, 6 mM MgCl2, 10 mM NaCl, 20 mM HEPES pH 7.5, 5 mM dithiothreitol (DTT), 0.01% Triton X-100, 2 mM spermidine, rNTPs (0.5 mM each), 3 pmol of [α-32P]rUTP and 3 pmol of T7 RNAP (or Y639F mutant) in a final reaction volume of 40 µl. The mixtures were incubated for 15 min at 37°C. Then, transcription products were resolved by 9% denaturing polyacrylamide gel electrophoresis. Determination of termination efficiencies and analysis of transcript 3′ ends were performed as described previously (Boudvillain et al., 2002).
NAIM assays
Termination and run-off transcripts containing NαS modifications at random positions were prepared simultaneously in 40 µl transcriptions with the Y639F RNAP and in the absence of [α-32P]rUTP. Each transcription reaction was doped with a single NTPαS analog. The NTPαS concentrations were adjusted according to published protocols (Strobel and Shetty, 1997; Ortoleva-Donnelly et al., 1998a,b) to yield ∼5% incorporations per run-off transcript. At this level of modification, potential cooperative effects between multiple NαS substitutions within a single transcript can be neglected (Christian and Yarus, 1992; Strobel and Shetty, 1997). The transcription reactions were mixed with single-stranded M13 DNA (1 µg) and KCl (250 mM) before being loaded onto Microcon columns (100 kDa cut-off) that had been saturated with bovine serum albumin (BSA) as described by the manufacturer (Millipore). After centrifugation of the columns for 2 min at 10 000 r.p.m. in a minifuge (Gopal et al., 1999), the filtrates were collected and desalted by Sephadex G-50 (Sigma) chromatography. They were then treated with alkaline phosphatase before phenol extraction and ethanol precipitation. The transcripts in the pellet were labeled with [γ-32P]ATP and polynucleotide kinase and purified by 9% polyacrylamide gel electrophoresis under strong denaturing conditions (7 M urea and 30% formamide; 60°C). In this way, anomalous gel migrations due to folded terminator hairpins (Jeng et al., 1990; Kashlev and Komissarova, 2002) were eliminated for the T1 transcripts (data not shown). Transcripts were then treated with iodine (Boudvillain and Pyle, 1998) and the resulting cleavage products were analyzed by 9% denaturing polyacrylamide gel electrophoresis followed by Phosphorimager (ImageQuant) scanning.
Analysis of the NαS signals
Atoms and functional groups within the T1 transcripts (between positions 30 and 88) have been probed with the following NTPαS analogs: parental rNTPαS, 2′-deoxy-NTPαS (dNTPαS), inosine-TPαS (ITPαS), N2-methyl-GTPαS (mGTPαS), purine-TPαS (RTPαS), 2-aminopurine-TPαS (2-APTPαS) and 7-deaza-RTPαS (C7-ATPαS and C7-GTPαS). The NαS signals have been quantified as described previously (Ortoleva-Donnelly et al., 1998b). Briefly, NAIM effects were deduced from the intensities of individual bands after normalization for differences in loading of the gel lanes. The NαS interference (κ) at a given position is expressed as the ratio of normalized band intensities that were obtained for this position:
κ = [INαS(run-off)/INαS(termination)]/[IαS(run-off)/IαS(termination)]
where NαS and αS refer to the analog (such as inosine-αS) and parental (such as guanosine-αS) nucleotides, respectively (Ortoleva-Donnelly et al., 1998b). A κ value >1 indicates a position of NαS incorporation that is detrimental to the termination process, whereas κ < 1 reveals a modification that favors termination. Because the selection stringency cannot be easily tuned in our NAIM assays (see Macdonald et al., 1993), only interference effects consistently observed in 3–4 independent experiments (SD <20%) were considered significant. Moreover, we used a discrimination factor (λ) to facilitate data analysis. First, κ values that were deviating by >2 SDs from the mean of the corresponding data set were excluded before another calculation of the SD. Then for every κ value of the data set, the discrimination factor was defined as:
λ = (1 – κ)/SD when κ > 1
λ = (1/κ – 1)/SD when κ < 1
Thus, λ normalizes NAIM signals for varying experimental quality and population extent among the different data sets and provides identical intensity scales for favorable (λ > 0) and detrimental (λ < 0) effects on transcription termination. Finally, positions of weak (2.5<|λ|<3), moderate (3<|λ|<5) and strong (|λ|>5) effects were identified (P ≤ 0.0124).
Acknowledgments
Acknowledgements
We thank William T.McAllister for the gift of the Y639F mutant of T7 RNAP, and Irina Artsimovitch for critical reading of the manuscript. This research was supported by the Association pour la Recherche sur le Cancer (grant no. 5560), the Ligue contre le Cancer (Loiret) and the ANRS (grant no. 02003).
References
- Boudvillain M. and Pyle,A.M. (1998) Defining functional groups, core structural features and inter-domain tertiary contacts essential for group II intron self-splicing: a NAIM analysis. EMBO J., 17, 7091–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudvillain M., de Lencastre,A. and Pyle,A.M. (2000) A tertiary interaction that links active-site domains to the 5′ splice site of a group II intron. Nature, 406, 315–318. [DOI] [PubMed] [Google Scholar]
- Boudvillain M., Schwartz,A. and Rahmouni,A.R. (2002) Limited topological alteration of the T7 RNA polymerase active center at intrinsic termination sites. Biochemistry, 41, 3137–3146. [DOI] [PubMed] [Google Scholar]
- Cheng S.W., Lynch,E.C., Leason,K.R., Court,D.L., Shapiro,B.A. and Friedman,D.I. (1991) Functional importance of sequence in the stem–loop of a transcription terminator. Science, 254, 1205–1207. [DOI] [PubMed] [Google Scholar]
- Christian E.L. and Yarus,M. (1992) Analysis of the role of phosphate oxygens in the group I intron from Tetrahymena. J. Mol. Biol., 228, 743–758. [DOI] [PubMed] [Google Scholar]
- d’Aubenton Carafa Y., Brody,E. and Thermes,C. (1990) Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem–loop structures. J. Mol. Biol., 216, 835–858. [DOI] [PubMed] [Google Scholar]
- Freier S.M. and Altmann,K.H. (1997) The ups and downs of nucleic acid duplex stability: structure–stability studies on chemically modified DNA:RNA duplexes. Nucleic Acids Res., 25, 4429–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish G. and Eckstein,F. (1988) DNA and RNA sequence determination based on phosphorothioate chemistry. Science, 240, 1520–1522. [DOI] [PubMed] [Google Scholar]
- Gopal V., Brieba,L.G., Guajardo,R., McAllister,W.T. and Sousa,R. (1999) Characterization of structural features important for T7 RNAP elongation complex stability reveals competing complex conformations and a role for the non-template strand in RNA displacement. J. Mol. Biol., 290, 411–431. [DOI] [PubMed] [Google Scholar]
- Guex N. and Peitsch,M.C. (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis, 18, 2714–2723. [DOI] [PubMed] [Google Scholar]
- Gusarov I. and Nudler,E. (1999) The mechanism of intrinsic transcription termination. Mol. Cell, 3, 495–504. [DOI] [PubMed] [Google Scholar]
- Gusarov I. and Nudler,E. (2001) Control of intrinsic transcription termination by N and NusA: the basic mechanisms. Cell, 107, 437–449. [DOI] [PubMed] [Google Scholar]
- Hartvig L. and Christiansen,J. (1996) Intrinsic termination of T7 RNA polymerase mediated by either RNA or DNA. EMBO J., 15, 4767–4774. [PMC free article] [PubMed] [Google Scholar]
- He B., Rong,M., Lyakhov,D., Gartenstein,H., Diaz,G., Castagna,R., McAllister,W.T. and Durbin,R.K. (1997) Rapid mutagenesis and purification of phage RNA polymerases. Protein Expr. Purif., 9, 142–151. [DOI] [PubMed] [Google Scholar]
- He B., Kukarin,A., Temiakov,D., Chin-Bow,S.T., Lyakhov,D.L., Rong,M., Durbin,R.K. and McAllister,W.T. (1998) Characterization of an unusual, sequence-specific termination signal for T7 RNA polymerase. J. Biol. Chem., 273, 18802–18811. [DOI] [PubMed] [Google Scholar]
- Hermann T. and Patel,D.J. (1999) Stitching together RNA tertiary architectures. J. Mol. Biol., 294, 829–849. [DOI] [PubMed] [Google Scholar]
- Huang Y., Beaudry,A., McSwiggen,J. and Sousa,R. (1997) Determinants of ribose specificity in RNA polymerization: effects of Mn2+ and deoxynucleoside monophosphate incorporation into transcripts. Biochemistry, 36, 13718–13728. [DOI] [PubMed] [Google Scholar]
- Jeng S.T., Gardner,J.F. and Gumport,R.I. (1990) Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J. Biol. Chem., 265, 3823–3830. [PubMed] [Google Scholar]
- Kashlev M. and Komissarova,N. (2002) Transcription termination: primary intermediates and secondary adducts. J. Biol. Chem., 277, 14501–14508. [DOI] [PubMed] [Google Scholar]
- Komissarova N., Becker,J., Solter,S., Kireeva,M. and Kashlev,M. (2002) Shortening of RNA:DNA hybrid in the elongation complex of RNA polymerase is a prerequisite for transcription termination. Mol. Cell, 10, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Landick R. (2001) RNA polymerase clamps down. Cell, 105, 567–570. [DOI] [PubMed] [Google Scholar]
- Lyakhov D.L., He,B., Zhang,X., Studier,F.W., Dunn,J.J. and McAllister,W.T. (1997) Mutant bacteriophage T7 RNA polymerases with altered termination properties. J. Mol. Biol., 269, 28–40. [DOI] [PubMed] [Google Scholar]
- Macdonald L.E., Zhou,Y. and McAllister,W.T. (1993) Termination and slippage by bacteriophage T7 RNA polymerase. J. Mol. Biol., 232, 1030–1047. [DOI] [PubMed] [Google Scholar]
- Macdonald L.E., Durbin,R.K., Dunn,J.J. and McAllister,W.T. (1994) Characterization of two types of termination signal for bacteriophage T7 RNA polymerase. J. Mol. Biol., 238, 145–158. [DOI] [PubMed] [Google Scholar]
- McDowell J.C., Roberts,J.W., Jin,D.J. and Gross,C. (1994) Determination of intrinsic transcription termination efficiency by RNA polymerase elongation rate. Science, 266, 822–825. [DOI] [PubMed] [Google Scholar]
- Mooney R.A., Artsimovitch,I. and Landick,R. (1998) Information processing by RNA polymerase: recognition of regulatory signals during RNA chain elongation. J. Bacteriol., 180, 3265–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller U., Schubel,H., Sprinzl,M. and Heinemann,U. (1999) Crystal structure of acceptor stem of tRNA(Ala) from Escherichia coli shows unique G·U wobble base pair at 1.16 Å resolution. RNA, 5, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K.S. and Darst,S.A. (2003) Bacterial RNA polymerases: the wholo story. Curr. Opin. Struct. Biol., 13, 31–39. [DOI] [PubMed] [Google Scholar]
- Ortoleva-Donnelly L., Kronman,M. and Strobel,S.A. (1998a) Identifying RNA minor groove tertiary contacts by nucleotide analogue interference mapping with N2-methylguanosine. Biochemistry, 37, 12933–12942. [DOI] [PubMed] [Google Scholar]
- Ortoleva-Donnelly L., Szewczak,A.A., Gutell,R.R. and Strobel,S. (1998b) The chemical basis of adenosine conservation throughout the Tetrahymena ribozyme. RNA, 4, 498–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rife J.P., Cheng,C.S., Moore,P.B. and Strobel,S.A. (1998) N2-methylguanosine is iso-energetic with guanosine in RNA duplexes and GNRA tetraloops. Nucleic Acids Res., 26, 3640–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaLucia J.J., Kierzek,R. and Turner,D.H. (1992) Context dependence of hydrogen bond free energy revealed by substitutions in an RNA hairpin. Science, 256, 217–219. [DOI] [PubMed] [Google Scholar]
- Steitz T.A. (1998) A mechanism for all polymerases. Nature, 391, 231–232. [DOI] [PubMed] [Google Scholar]
- Strobel S.A. (1999) A chemogenetic approach to RNA function/structure analysis. Curr. Opin. Struct. Biol., 9, 346–352. [DOI] [PubMed] [Google Scholar]
- Strobel S.A. and Shetty,K. (1997) Defining the chemical groups essential for Tetrahymena group I intron function by nucleotide analog interference mapping. Proc. Natl Acad. Sci. USA, 94, 2903–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N., Nakano,M. and Nakano,S. (2000) Thermodynamics–structure relationship of single mismatches in RNA–DNA duplexes. Biochemistry, 39, 11270–11281. [DOI] [PubMed] [Google Scholar]
- Szewczak A.A., Ortoleva-Donnelly,L., Ryder,S.P., Moncoeur,E. and Strobel,S.A. (1998) A minor groove RNA triple helix within the catalytic core of a group I intron. Nat. Struct. Biol., 5, 1037–1042. [DOI] [PubMed] [Google Scholar]
- Tahirov T.H., Temiakov,D., Anikin,M., Patlan,V., McAllister,W.T., Vassylyev,D.G. and Yokoyama,S. (2002) Structure of a T7 RNA polymerase elongation complex at 2.9 Å resolution. Nature, 420, 43–50. [DOI] [PubMed] [Google Scholar]
- Temiakov D., Anikin,M. and McAllister,W.T. (2002) Characterization of T7 RNA polymerase transcription complexes assembled on nucleic acid scaffolds. J. Biol. Chem., 277, 47035–47043. [DOI] [PubMed] [Google Scholar]
- Toulokhonov I., Artsimovitch,I. and Landick,R. (2001) Allosteric control of RNA polymerase by a site that contacts nascent RNA hairpins. Science, 292, 730–733. [DOI] [PubMed] [Google Scholar]
- Wilson K.S. and von Hippel,P.H. (1994) Stability of Escherichia coli transcription complexes near an intrinsic terminator. J. Mol. Biol., 244, 36–51. [DOI] [PubMed] [Google Scholar]
- Wilson K.S., Conant,C.R. and von Hippel,P.H. (1999) Determinants of the stability of transcription elongation complexes: interactions of the nascent RNA with the DNA template and the RNA polymerase. J. Mol. Biol., 289, 1179–1194. [DOI] [PubMed] [Google Scholar]
- Woody A.Y., Eaton,S.S., Osumi-Davis,P.A. and Woody,R.W. (1996) Asp537 and Asp812 in bacteriophage T7 RNA polymerase as metal ion-binding sites studied by EPR, flow-dialysis and transcription. Biochemistry, 35, 144–152. [DOI] [PubMed] [Google Scholar]
- Yarnell W.S. and Roberts,J.W. (1999) Mechanism of intrinsic transcription termination and antitermination. Science, 284, 611–615. [DOI] [PubMed] [Google Scholar]
- Yin Y.W. and Steitz,T.A. (2002) Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science, 298, 1387–1395. [DOI] [PubMed] [Google Scholar]