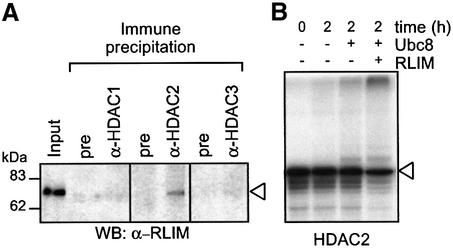
Fig. 5. RLIM interacts with HDAC2 and induces poly-ubiquitination in vitro. (A) Interaction of HDACs 1, 2 and 3 with RLIM was tested by co-immunoprecipitation from whole-cell extracts of HEK293T cells that had been pretreated with the proteasome inhibitor ALLN (25 µM) for 4 h. Immunoprecipitations efficiently depleted HDACs from the cell extracts (not shown) and coprecipitated RLIM was detected by western blot analysis. Control immunoprecipitations were performed with non-immune serum. The left lane shows 5% of the extract used for immunoprecipitation. (B) In vitro translated [35S]methionine-labeled HDAC2 was incubated for 2 h in buffer with E1 ubiquitin ligase and ubiquitin only, or in reactions containing the recombinantly expressed E2 ubiquitin ligase Ubce8 without or with the E3 ligase RLIM. For control (left lane), the reaction was stopped immediately after addition of ubiquitin and E1 ligase. Loss of input HDAC2 and appearance of high molecular weight radiolabeled proteins were determined by SDS–PAGE followed by autoradiography.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.