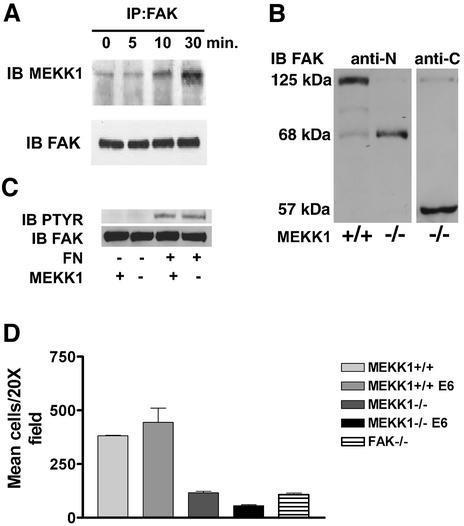
Fig. 4. MEKK1 and FAK form a complex in EGF-stimulated fibroblasts. (A) Wild-type mouse embryo fibroblasts were treated with 100 ng/ml EGF for the indicated times. After cell lysis, endogenous FAK was immunoprecipitated with anti-FAK antibody, and associated MEKK1 detected by MEKK1 immunoblotting. Total immunoprecipitated FAK was measured by anti-FAK immunoblotting using the same blots as that for MEKK1 analysis. (B) Wild-type (MEKK1+/+) and MEKK1–/– fibroblasts stably expressing papilloma virus E6/E7 proteins were immunoblotted for FAK protein expression using an antibody recognizing the N- or C-terminal domain of FAK. FAK is a 125 kDa protein with N- and C-terminal cleavage fragments of 68 and 57 kDa, respectively. (C) Serum-starved MEKK1+/+ or MEKK1–/– MEFs were allowed to adhere to fibronectin-coated bacterial plates for 30 min, and then lysed. Endogenous FAK was immunoprecipitated with anti-FAK antibodies, and the level of tyrosine-phosphorylated FAK determined by phosphotyrosine immunoblotting. The membrane was then stripped and total FAK was assessed by FAK immunoblotting. (D) MEKK1+/+ and MEKK1–/– fibroblasts ± E6/E7 protein expression were analyzed for migration in Transwell assays using 5% FBS as described for Figure 2A. *Inhibition of MEKK1–/– E6/E7 cell migration is statistically significant relative to FAK–/– cells (P < 0.05) and MEKK1–/– cells (P < 0.001).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.