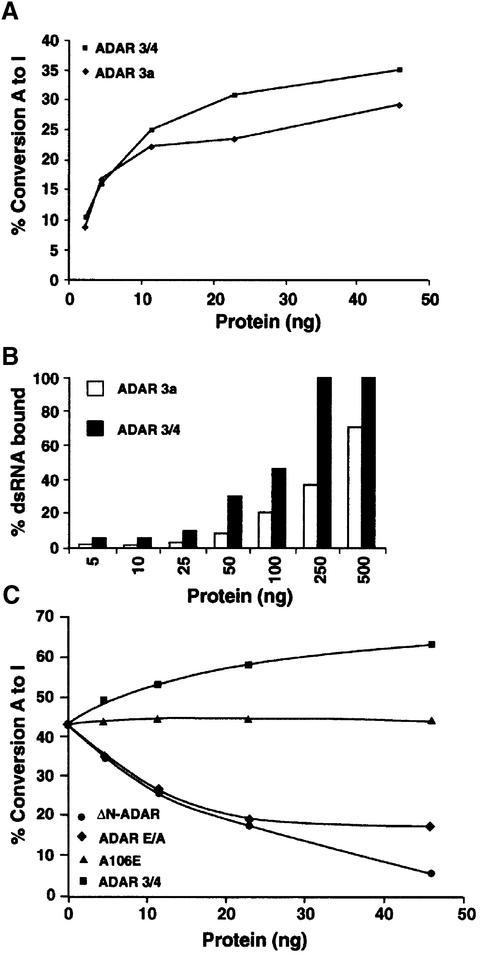
Fig. 6. Inactive ADAR that retains the minimum dimerization domain downregulates ADAR activity in vitro. (A) A graph representing the editing activity of purified ADAR 3a and 3/4 on 32P-labelled dsRNA. The 3/4 isoform was more active than the 3a isoform. The protein amounts used are indicated. (B) A graph representing the binding of purified 3a and 3/4 proteins to 32P-labelled dsRNA. The 3/4 binding saturated at 250 ng, whereas the 3a isoform bound less dsRNA under the same experimental conditions. (C) In vitro editing assay was performed with a constant amount of ADAR 3a (7 ng). Increasing amounts of inactive 3/4-E/A (diamonds), or 3/4 wild-type (squares) or A106E (triangles) or ΔN-ADAR (circles) were added to this mixture; the protein amount that was added is indicated. A106E is a negative control as it cannot dimerize or bind to dsRNA, whereas ADAR 3/4 is a positive control.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.