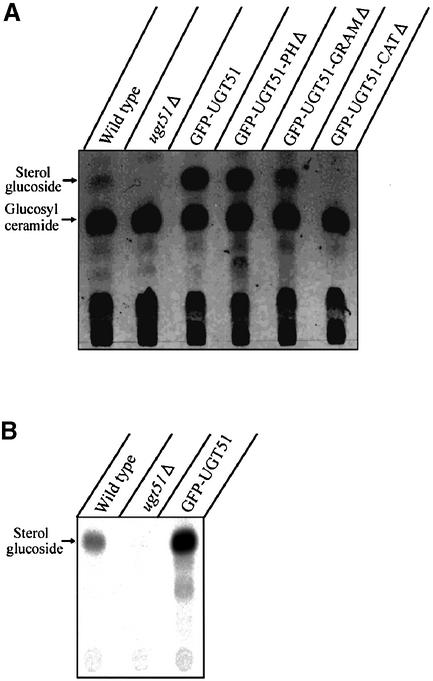
Fig. 3. The catalytic domain, but not the PH or GRAM domains, is essential for SG biosynthesis by Ugt51. Methanol-grown P.pastoris cells were shifted to glucose medium and harvested after 2 h. (A) Total lipids were extracted and subjected to thin-layer chromatography. Low levels of SG were detected in wild-type cells, whereas the knock-out strain ugt51Δ was completely devoid of this lipid. The expression of GFP-tagged Ugt51 variants under the control of the strong AOX1 promoter led to the accumulation of considerable amounts of SG, even in strains expressing Ugt51 devoid of the GRAM or PH domain (strains GFP–UGT51, GFP–UGT51-PHΔ and GFP–UGT51-GRAMΔ). Only the deletion of the catalytic domain diminished SG synthesis (strain GFP–UGT51-CATΔ). (B) In vitro enzyme assays were performed to detect the sterol glucosyltransferase activity. Wild-type cells displayed low, but significant activity, which was absent in ugt51Δ cells. The catalytic activity in strain GFP–UGT51 was 30× higher than that of the wild type due to the strong expression by the AOX1 promoter.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.