Abstract
Nek2A is a cell cycle-regulated kinase of the never in mitosis A (NIMA) family that is highly enriched at the centrosome. One model for Nek2A function proposes that it regulates cohesion between the mother and daughter centriole through phosphorylation of C-Nap1, a large coiled-coil protein that localizes to centriolar ends. Phosphorylation of C-Nap1 at the G2/M transition may trigger its displacement from centrioles, promoting their separation and subsequent bipolar spindle formation. To test this model, we generated tetracycline-inducible cell lines overexpressing wild-type and kinase-dead versions of Nek2A. Live cell imaging revealed that active Nek2A stimulates the sustained splitting of interphase centrioles indicative of loss of cohesion. However, this splitting is accompanied by only a partial reduction in centriolar C-Nap1. Strikingly, induction of kinase-dead Nek2A led to formation of monopolar spindles with unseparated spindle poles that lack C-Nap1. Furthermore, kinase-dead Nek2A interfered with chromosome segregation and cytokinesis and led to an overall change in the DNA content of the cell population. These results provide the first direct evidence in human cells that Nek2A function is required for the correct execution of mitosis, most likely through promotion of centrosome disjunction. However, they suggest that loss of centriole cohesion and C-Nap1 displacement may be distinct mitotic events.
INTRODUCTION
A centrosome is classically depicted as having two orthogonally positioned cylindrical centrioles surrounded by a matrix of fibrous and globular proteins that constitute the pericentriolar material (PCM). However, this simple description belies the complicated structural changes that occur at the centrosome during progression through the cell cycle (Doxsey, 2001; Hinchcliffe and Sluder, 2001; Meraldi and Nigg, 2002). After a mitotic division, cells inherit a pair of morphologically distinct centrioles, called the mother and daughter (Marshall, 2001). At some point early in the cell cycle, these undergo disorientation, whereby they lose the orthogonal arrangement and become more obviously separated. The precise timing of centriole disorientation is a matter of debate and may be cell type dependent. During S and G2, centriole duplication occurs with procentrioles that can only clearly be detected by electron microscopy elongating at a perpendicular angle from the surface of mother and daughter centrioles. As cells enter mitosis, the original mother and daughter centriole become detached from one another in an event termed centrosome disjunction (Hinchcliffe and Sluder, 2001). The two pairs of duplicated centrioles then separate toward opposite ends of the cell to facilitate mitotic spindle formation. The PCM also undergoes structural reorganization during cell cycle progression, most notably upon entry into mitosis when additional proteins are recruited and there is a change in microtubule nucleating capacity (Khodjakov and Rieder, 1999). The remodeling of the PCM as cells enter mitosis is referred to as centrosome maturation.
The changes in centriole and PCM organization that occur during the centrosome duplication cycle have been well documented by electron and light microscopy of fixed cells and isolated centrosomes. However, it is only with the recent application of green fluorescent protein (GFP) technology, coupled with live cell imaging, that it has become apparent that centrioles are not static organelles within the cytoplasm. On the contrary, centrioles exhibit dramatic and extensive movements that may be critical for initiating specific cell cycle events as well as for determining the distribution of the microtubule network. The mother centriole, for example, migrates toward the midbody during late mitosis in an event that precedes, and may be necessary for, cell abscission (Piel et al., 2001). Meanwhile in G1, the mother centriole remains close to the center of the cell, whereas the daughter oscillates around it visiting different regions of the cytoplasm (Piel et al., 2000). The extent of these movements varies according to cell type, with centrioles exhibiting a greater degree of movement in L929 and NIH 3T3 cells, for instance, than HeLa cells. In HeLa cells, centrioles remain in relative proximity throughout interphase raising the possibility that proteinaceous material, perhaps part of the PCM, acts as a physical tether between mother and daughter centriole after disorientation. In fact, even in those cell types where centrioles migrate far apart, some sort of intercentriolar connection seems to be present because the G1 movements of the two centrioles are correlated and, in S and G2, the centrioles move back closer together and become less dynamic (Piel et al., 2000).
Perhaps the most persuasive evidence that a physical linkage is established between mother and daughter centriole after disorientation is the fact that centrioles remain paired in isolated centrosome preparations (Bornens and Moudjou, 1999). Electron dense material has been observed between the mother and daughter centrioles of isolated centrosomes, as well as between the pair of basal bodies in Chlamydomonas, but the molecular nature of these connections remains a mystery (Bornens et al., 1987; Paintrand et al., 1992; Preble et al., 2000). Some progress, though, has been made into proteins that regulate centriolar cohesion. Nek2A is a never in mitosis A (NIMA)-related protein kinase that forms a complex with the catalytic subunit of protein phosphatase 1 (PP1) and a large coiled-coil protein called C-Nap1 (Fry, 2002). Nek2A can phosphorylate itself, PP1 and C-Nap1, whereas PP1 can dephosphorylate both Nek2A and C-Nap1 (Fry et al., 1998b, 1999; Helps et al., 2000). Transient overexpression of active Nek2A kinase or microinjection of C-Nap1 antibodies leads to premature splitting of the mother and daughter centrioles, suggesting that these treatments either stimulate centriole dynamics or somehow modify the intercentriolar linkage (Fry et al., 1998a; Mayor et al., 2000). Coexpression of PP1 with Nek2A suppresses centriole splitting, whereas ectopic expression of inhibitor protein 2, a physiological inhibitor of PP1, stimulates splitting (Meraldi and Nigg, 2001; Eto et al., 2002). Together, these studies support a model in which centriole cohesion is carefully modulated by opposing kinase and phosphatase activities.
Nek2 and C-Nap1 both localize to the proximal ends of centrioles, which would be consistent with their forming an anchoring site for an intercentriolar linkage (Fry et al., 1998b; Mayor et al., 2000). Moreover, C-Nap1 is displaced from spindle poles in mitosis at a time when it is hyperphosphorylated in vivo (Mayor et al., 2002). These observations provide the basis for a model in which Nek2A promotes centrosome disjunction through phosphorylation-induced displacement of C-Nap1 from centriolar ends (Mayor et al., 1999; Fry, 2002). If this model were correct then overexpression of a catalytically-inactive Nek2A protein might be expected to interfere with centrosome disjunction and, subsequently, mitotic progression. However, in transient transfection experiments such phenotypes have been difficult to observe. We therefore decided to generate stable cell lines expressing active and kinase-dead Nek2A from tetracycline-inducible promoters. Using these cell lines, we have, for the first time, demonstrated a dominant-negative phenotype for kinase-dead Nek2A with respect to centrosome disjunction, spindle formation, and chromosome segregation. These results provide the strongest evidence yet that Nek2 has an important function in coordinating centrosome structure and function with mitotic progression alongside other protein kinases such as Cdk1, Plk1, and Aurora-A (Nigg, 2001).
MATERIALS AND METHODS
Cell Culture and Transfections
T-REx-U2OS cells (Invitrogen, Carlsbad, CA), as well as derived cell lines, were grown at 37°C in a 5% CO2 atmosphere in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin (100 IU/ml and 100 μg/ml, respectively). Hygromycin B (200 μg/ml) was added to maintain the integrated vector expressing the Tet repressor protein. Transfection of expression plasmids was performed using calcium phosphate as described previously (Fry and Faragher, 2001). To induce and maintain expression from tetracycline-responsive promoters, tetracycline or doxycycline (1 μg/ml) was added to the culture medium every 24 or 48 h, respectively. To induce cytokinesis failure, cytochalasin D (0.6 μg/ml) was added.
Plasmid Constructions
Generation of pEGFP-Nek2A has been described previously (Hames and Fry, 2002). pEGFP-Nek2A-K37R was made by excision of Nek2A-K37R from pGEM-Nek2-K37R (Fry et al., 1995) on a NaeI-BamHI fragment and inserting it into a pEGFP-C1 vector (BD Biosciences Clontech, Palo Alto, CA) modified to include a T7 promoter upstream of the enhanced green fluorescent protein (EGFP) coding region. To generate vectors for inducible expression of C-terminal myc-His–tagged proteins, full-length cDNAs encoding wild-type Nek2A or Nek2A-K37R were excised from pEGFP-Nek2A or pEGFP-Nek2A-K37R, respectively, as SphI-XhoI blunted fragments and subcloned into the pcDNA4/TO/myc-HisA inducible eukaryotic expression vector (Invitrogen) cut with XhoI and blunted. To generate vectors for inducible expression of N-terminal EGFP–tagged proteins, EGFP-Nek2A, wild-type, or K37R, was excised from pEGFP-Nek2A or pEGFP-Nek2A-K37R, respectively, on an AgeI (blunted)-XbaI fragment and subcloned into the pcDNA4/TO inducible eukaryotic expression vector (Invitrogen) cut with EcoRV and XbaI.
Generation of Tetracycline-inducible Cell Lines
Stable cell lines were generated by introducing the relevant construct into a 10-cm dish of T-REx-U2OS cells by using calcium phosphate-mediated transfection. After 24 h, cells were washed and incubated in selective media containing Zeocin (400 μg/ml; Invitrogen) and hygromycin B (200 μg/ml) until foci containing ∼50 –100 cells were detected (∼3–4 wk). Culture dishes were then washed with 1× phosphate-buffered saline (PBS) before picking foci by using paper cloning discs (Sigma-Aldrich, St. Louis, MO; Z37, 443-1). Each disk was soaked in 1× PBS containing 0.5 mM EDTA and then placed over the foci of cells for 5–10 min at 37°C. Discs were then transferred into individual wells of a 24-well plate and selective medium added. After attachment of cells to plastic culture wells, cloning discs were removed and individual clones expanded in selective media before testing for protein expression by Western blotting and fluorescence microscopy.
Cell Extraction, Protein Electrophoresis, and Western Blotting
To prepare cell extracts, cells were washed once with ice-cold 1× PBS before direct lysis on the dish in hot (95°C) SDS-gel loading buffer. Lysed cells were collected from the plate with a cell scraper and heated to 95°C for 10 min. Chromosomal DNA was sheared by passage through a 27-gauge needle before centrifugation for 10 min. Protein gel electrophoresis and Western blotting was performed as described previously (Fry et al., 1998b). Primary antibodies used were anti-Nek2 (1 μg/ml; Fry et al., 1999) and anti-phospho-H3 (1 μg/ml; Upstate Biotechnology, Lake Placid, NY), whereas secondary antibodies were alkaline phosphatase-conjugated anti-rabbit or anti-mouse IgGs (1:7500; Promega, Madison, WI).
Fixed Cell Microscopy
Immunofluorescence microscopy was performed as described previously (Fry et al., 1998a; Fry and Faragher, 2001), except for GT335 staining where cells were first detergent extracted according to MacRae et al. (1990). Primary antibodies used were anti-Nek2 (1 μg/ml; Zymed, South San Francisco, CA; Fry et al., 1999), anti-C-Nap1 (1 μg/ml; R63 anti-C-term antibody; Fry et al., 1998b), antimyc (9E10; undiluted supernatant), anti-Plk1 (10 μg/ml; Upstate Biotechnology), anti-polyglutamylated tubulin (GT335; 1:200), α-cytokeratin 18 caspase cleavage product (M30 CytoDEATH; Roche Diagnostics, Indianapolis, IN), anti-γ-tubulin (0.15 μg/ml; Sigma-Aldrich), and anti-α-tubulin (0.3 μg/ml; Sigma-Aldrich). Secondary antibodies used were Alexa Fluor 488 goat anti-rabbit and rabbit anti-mouse IgGs (1 μg/ml; Molecular Probes), donkey anti-rabbit and sheep anti-mouse biotinylated whole antibodies (1:100; Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). Streptavidin Texas Red (1:200; Amersham Biosciences UK) was used to detect biotinylated antibodies, whereas DNA was stained with Hoechst 33258 (0.2 μg/ml; Calbiochem, San Diego, CA). Fluorescence images were captured on a Nikon TE300 inverted microscope using an ORCA ER charge-couple device camera (Hamamatsu, Shizuoka, Japan) by using Openlab 3.09 software (Improvision, Coventry, United Kingdom) and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). Digital deconvolution was performed by capturing optical z-sections at 0.1-μm steps through the cell of interest using a high-speed Piezo focus drive device (Orbit II). Volume deconvolution (Openlab 3.09; Improvision) by using five nearest neighbors was used on each z-section before display as maximum intensity projections.
Centrosome Intensity Measurements
Cells were processed for indirect immunofluorescence microscopy by using anti-C-Nap1 or anti-γ-tubulin antibodies, as described above. Using constant exposure times and gain settings determined to be within the linear range of the camera, we captured fluorescence images of centrosomes and mitotic spindle poles. A 1.5-μm2 region of interest was positioned to encompass each centrosome and the mean intensity (integrated optical density) signal per pixel within this region of interest, minus background, was measured. Measurements were made on each centrosome in 20 cells for each condition and independent experiments performed three times.
Live Cell Imaging
For live cell microscopy, cells were grown on coverslips and induced with doxycycline as indicated in the text. Coverslips were then washed once with 1× PBS before mounting in a steel coverslip holder (made in house) in CO2-independent medium without glutamine (Invitrogen) but with doxycycline. The surface of the medium was overlaid with mineral oil (Sigma-Aldrich) to prevent evaporation. The steel coverslip holder was mounted in a Patch Slice MicroIncubator regulated at 37°C by a TC-202A temperature controller (Harvard Apparatus Medical Systems Research Products, supplied by Digitimer, Hertfordshire, United Kingdom). The Patch Slice MicroIncubator was placed on the microscope stage and images captured at 2- or 5-min intervals with the microscope system described above. At each time point, five optical sections (z-planes) at 0.5-μm intervals were captured using a high-speed Piezo focus drive (Orbit II) attached to a 60× oil objective (numerical aperture 1.4) and merged using Openlab software. Images were processed using Adobe Photoshop or converted to QuickTime movies.
Flow Cytometry
Cell cycle distributions were determined by measuring DNA content by flow cytometry after staining of cells with propidium iodide. Specifically, 1 × 106 cells were washed with 1× PBS and slowly resuspended in 1 ml of 70% ethanol (–20°C) before storage at –20°C. Fixed cells were pelleted and washed with 1× PBS before resuspension in 1 ml of DNA stain (0.02 mg/ml propidium iodide, 0.2 mg/ml RNase A in 1× PBS) and incubated overnight at 4°C. DNA content was measured using a FACScan II instrument (BD Biosciences, San Jose, CA) and analyzed using CellQuest.
RESULTS
Generation of Tetracycline-inducible Nek2A Cell Lines
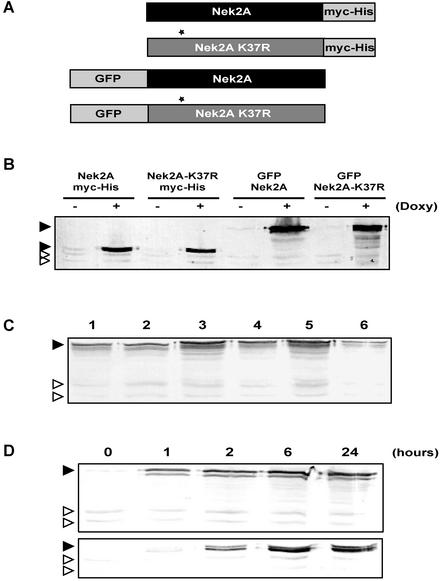
To address the role of Nek2A in centrosome organization and cell cycle progression, human U2OS cell lines were established with integrated copies of the Nek2A cDNA driven from tetracycline-responsive promoters. Four “Tet-ON” cell lines were generated comprising wild-type Nek2A or the catalytically-inactive (kinase-dead) Nek2A-K37R mutant with either an N-terminal enhanced GFP tag or a C-terminal myc-hexahistidine (myc-His) tag (Figure 1A). Western blotting of cell extracts with a Nek2 antibody revealed expression of a protein of the expected size in each cell line upon 24-h treatment with the tetracycline analog doxycycline (Figure 1B). Several independent clones were isolated for each cell line (Figure 1C; our unpublished data), but for this study, clones were used in which the GFP and myc-His–tagged proteins were induced to a level that was six- to eight- and four- to fivefold higher than endogenous Nek2A, respectively. Time courses of induction showed that recombinant proteins were expressed within 1 h of tetracycline addition and reached a maximal level by 6 h (Figure 1D).
Figure 1.
Generation of tetracycline-inducible Nek2A cell lines. (A) Schematic representation of the four Nek2A fusion proteins used for generation of tetracycline-inducible U2OS cell lines. Nek2A-K37R represents the catalytically inactive mutant kinase with a point mutation (asterisk) in the kinase domain. GFP, enhanced green fluorescent protein tag; Myc-His, myc epitope, and hexahistidine tag. (B) Western blot with α-Nek2 antibodies of extracts from each cell line after 24 h induction with (+) or without (–) doxycycline. (C) Western blot with α-Nek2 antibodies of six independent clones of the GFP-Nek2A-K37R cell line after 24-h doxycycline induction, indicating moderate clone to clone variation in recombinant protein expression level. (D) Western blot with α-Nek2 antibodies showing a time course of expression from a GFP-Nek2A (top) and Nek2A-K37R-myc-His (bottom) cell line after tetracycline induction (hours). In B, C, and D, filled arrowheads indicate recombinant Nek2A proteins; open arrowheads indicate migration of endogenous Nek2A (top band) and Nek2B (bottom band) proteins.
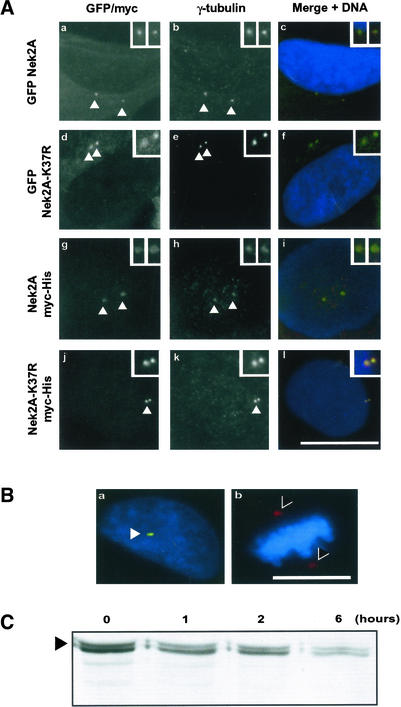
Fluorescence microscopy was performed on each cell line after 24-h induction. Exogenous Nek2A proteins primarily localized to the interphase centrosome as indicated by colocalization of the myc or GFP signal with γ-tubulin (Figure 2A). Isolation of centrosomes from these cells by sucrose gradient centrifugation also showed enrichment of recombinant proteins by Western blot (our unpublished data). Induction of wild-type Nek2A, either GFP or myc-His tagged, led to premature centriole splitting as judged by the loss of close juxtaposition of the two γ-tubulin–stained structures. This was not observed with the kinase-dead versions of Nek2A. Reassuringly, these results fall in line with those obtained in transient transfection experiments in which it was shown that active, but not inactive, Nek2A can induce centriole splitting (Fry et al., 1998a).
Figure 2.
Recombinant Nek2A localizes to centrosomes but not spindle poles. Cell lines were induced with doxycycline for 24 h before processing for fluorescence microscopy. GFP-Nek2A (a–c), GFP-Nek2A-K37R (d–f), Nek2A-myc-His (g–i), and Nek2A-K37R-myc-His (j–l). GFP signal (a and d), α-myc antibodies (g and j), anti-γ-tubulin antibodies (b, e, h, and k), and merged image of GFP or myc (green), γ-tubulin (red), and Hoechst 33258 (blue) (c, f, i, and l). Bar, 15 μm. Centrosomes (arrowheads) are shown at increased magnification in insets. (B) Merged images showing Nek2A-K37R-myc-His–induced cells in interphase (a) or metaphase (b) stained with antibodies against γ-tubulin (red) and myc (green). DNA is stained in blue. Recombinant Nek2A is detected at interphase centrosomes (arrowhead) but not mitotic spindle poles (>). Bar, 15 μm. (C) Western blot with anti-Nek2 antibodies of extracts from cells induced to express Nek2A-K37R-myc-His (arrowhead) for 24 h before addition of cycloheximide for the times indicated (hours).
On entry into mitosis, centrosome staining was lost (Figure 2B), supporting the finding that Nek2A is actively destroyed by the proteasome, rather than being transcriptionally down regulated, in early mitosis (Hames et al., 2001). Importantly, destruction led to loss of Nek2A from spindle poles and not simply from the noncentrosomal pool. In contrast to a recent report (Kim et al., 2002), no localization to mitotic chromosomes (Figure 2B, b) or the midbody (our unpublished data) was observed. By Western blotting for Nek2 protein in asynchronous cells incubated with cycloheximide (Figure 2C), the half-life of recombinant Nek2A-K37R-myc-His was determined to be very similar to endogenous Nek2A at ∼1 h (Hames et al., 2001). Thus, based upon localization, activity and stability measurements, these cell lines represent excellent new tools to study Nek2 function.
Analysis of Nek2A-induced Centriole Splitting in Live Cells
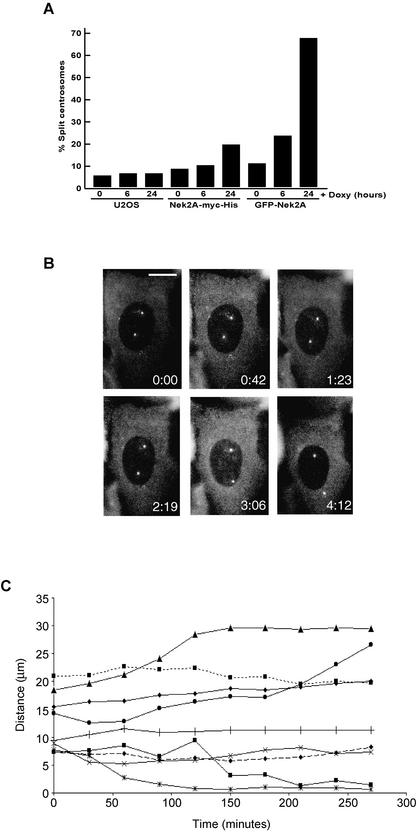
In certain cell types (e.g., L929) centrioles exhibit significant mobility during interphase (Piel et al., 2000), but in others the mother and daughter centriole remain in proximity. We calculated that in the parental U2OS cell line, although two dots can almost invariably be distinguished, <10% cells (n >200) have centrioles that are >2 μm apart (Figure 3A). However, doxycycline-induced expression of GFP-Nek2A or Nek2A-myc-His for 24 h stimulated a premature splitting of centrioles in 67 and 19% cells (n > 200), respectively (Figure 3A). This correlates with the higher level of expression in the GFP-Nek2A cell line and supports the notion that splitting depends upon achieving a certain threshold of Nek2A activity that is presumably in excess of competing PP1 activity. Proteasomal destruction in G1 is likely to reduce the level of recombinant Nek2A below this threshold explaining why splitting is not observed in 100% of cells (Fry et al., 1998a; Meraldi and Nigg, 2001).
Figure 3.
Active Nek2A kinase induces loss of centrosome cohesion. (A) Percentage of cells in which γ-tubulin–stained centrosomes were separated by >2 μm is indicated for U2OS-T-REx, Nek2A-myc-His, or GFP-Nek2A cells treated with doxycycline for 0, 6, or 24 h. (B) Selected images from a time-lapse movie of a cell induced to express GFP-Nek2A for 24 h showing that centrosomes remain split for more than 4 h (times in hours:minutes indicated on each panel). Bar, 15 μm. (C) Intercentriolar distances (micrometers) were measured over time (minutes) on captured images from single cell time-lapse experiments as shown in B. Cells were selected for imaging if the intercentriolar distance was initially >5 μm. Results from nine experiments are shown.
To study Nek2A localization and centriole splitting in live cells, time-lapse microscopy was performed on cells that had been induced to express wild-type GFP-Nek2A for 24 h. GFP-Nek2A protein was clearly detected at the two centrioles in live cells, providing conclusive evidence that previous staining patterns on fixed cells were not localization artifacts (Figure 3B). In most cells, the residual GFP fluorescence not associated with centrioles was detected in the cytoplasm and was faintly punctate in nature (our unpublished data). By selecting cells that had widely spaced centrioles (>5 μm) at the start of filming, it became apparent that Nek2A-induced splitting is not a transient event. In each cell, centrioles were observed for at least 3 h and, in the majority of cases (82%, n = 11), centrioles remained far apart and sometimes moved further apart (Figure 3, B and C). Moreover, fine movements of the individual centrioles seemed independent, implying that, if they were still linked, the connection was not under obvious tension (our unpublished data). Only in two cells (18%) did split centrioles return to a separation distance of <5 μm. However, as the initial intercentriolar distance was <10 μm in these cells, it is possible that any intercentriolar connections had not yet been fully dismantled. In summary, live cell imaging demonstrates that Nek2A overexpression stimulates either loss or relaxation of the attachment between mother and daughter centrioles.
C-Nap1 Is Only Partially Displaced from Split Centrioles
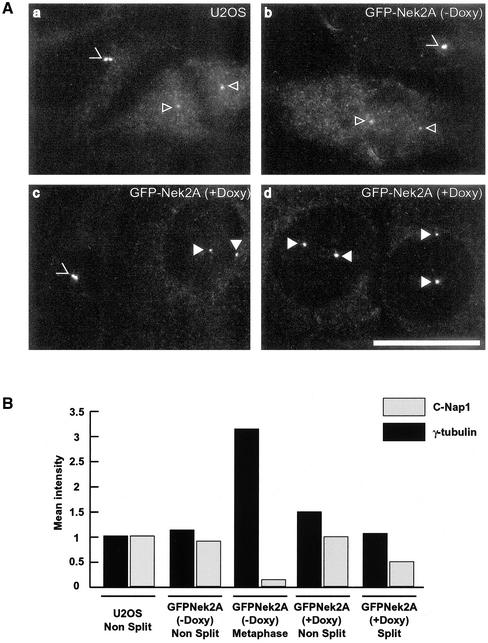
The hypothesis has been put forward that phosphorylation of C-Nap1 by Nek2 at the onset of mitosis triggers its displacement from the proximal ends of centrioles which, in turn, leads to centrosome disjunction (Mayor et al., 2002). We therefore examined whether premature splitting of interphase centrioles by active Nek2A kinase was accompanied by a similar loss of the C-Nap1 protein to that seen in normal mitosis. U2OS cells were fixed in cold methanol and processed by indirect immunofluorescence microscopy with anti-C-Nap1 antibodies. Quantitative imaging of parental U2OS cells (Figure 4A, a) or the GFP-Nek2A cell line without induction (Figure 4A, b) indicated a 10-fold reduction in C-Nap1 protein on mitotic spindle poles compared with unsplit centrosomes in neighboring interphase cells (Figure 4B). Imaging of C-Nap1 in the GFP-Nek2A cell line after doxycycline induction revealed that interphase cells with split centrioles had reduced C-Nap1 abundance, although not to the same extent as at mitotic spindle poles (Figure 4A, c and d). Quantitation revealed only a twofold decrease in C-Nap1 abundance on split, compared with unsplit, centrioles (Figure 4B). To determine the specificity of this response, similar quantitative imaging was performed on γ-tubulin (Figure 4B). In contrast to C-Nap1, the abundance of γ-tubulin at mitotic spindle poles was approximately threefold higher than at interphase centrosomes in agreement with previous studies showing recruitment of γ-tubulin during centrosome maturation (Khodjakov and Rieder, 1999). γ-Tubulin levels though were relatively unchanged between split and unsplit centrioles, suggesting that the moderate displacement of C-Nap1 protein is a specific effect and not due to general disintegration of the centrosome structure. However, whether the twofold reduction in C-Nap1 protein is sufficient to trigger the centriole splitting remains to be proven.
Figure 4.
C-Nap1 is still present on Nek2A-induced split centrosomes. (A) U2OS T-REx cells (a) or GFP-Nek2A cells without (b) or with 24-h doxycycline induction (c and d) were fixed and immunostained with anti-C-Nap1 antibodies. Images were captured under identical conditions to allow comparison of C-Nap1 signal intensity. Unsplit (>) and split (filled arrowheads) centrosomes in interphase centrosomes, and mitotic spindle poles (open arrowheads) are indicated. Bar, 15 μm. (B) Abundance of C-Nap1 and γ-tubulin at centrosomes was calculated by measuring mean pixel intensities as indicated in MATERIALS AND METHODS. Centrosomes in 20 cells for each condition were imaged, quantitated, and normalized to the intensity of unsplit interphase centrosomes in the parental U2OS cell line (given an arbitrary value of 1.0). Results show the mean of three independent experiments in which all cells were fixed at the same time and immunostained with C-Nap1 antibodies under identical conditions.
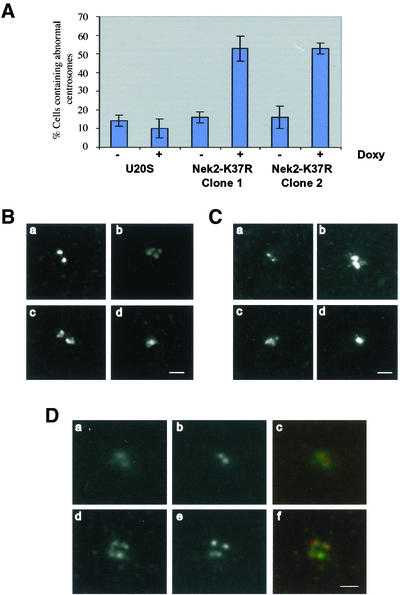
Kinase-Dead Nek2A Induces Accumulation of Multiple Centrosomes
Transient transfection of kinase-dead Nek2A had not revealed an obvious mitotic defect (Fry et al., 1998a). However, in those experiments it was difficult to detect transfected mitotic cells. The reason for this is clear now that we know that Nek2A is destroyed soon after mitotic entry (Hames et al., 2001). In contrast, kinase-dead Nek2A was capable of suppressing nocodazole-induced centriole splitting, implying that it could exert a dominant-negative activity (Meraldi and Nigg, 2001). We therefore decided to reexamine the consequences of overexpressing kinase-dead Nek2A by using the tetracycline-inducible cell lines. Careful examination of centrosomes in the Nek2A-K37R-myc-His cell line first revealed that >50% of cells (n = 500) had centrosomes that were abnormal in appearance, i.e., they no longer consisted of just two discrete dots of ∼1 μm in diameter (Figure 5A). This was evident not only after immunostaining with anti-Nek2 antibodies (Figure 5B) but also with anti-γ-tubulin (Figure 5C) and anti-C-Nap1 (our unpublished data) antibodies. In some cases, only one or two structures could be distinguished, although these were often much larger than a normal centrosome and irregular in shape (Figure 5, B and C, d). In other cases, however, there were clearly more than two centrosomes that again were not necessarily of normal appearance (Figure 5, B and C, b and c). These phenotypes were strictly dependent upon expression of the recombinant protein because they were not observed in the parental cell line or in the mutant cell line in the absence of tetracycline. Moreover, the abnormal phenotypes were evident in more than one clone of the Nek2A-K37R-myc-His cell line. Microtubule regrowth assays after cold-induced microtubule depolymerization indicated that all of these centrosomes were still capable of efficient microtubule nucleation (our unpublished data).
Figure 5.
Induction of centrosome abnormalities in cells expressing kinase-dead Nek2A. (A) Percentage of cells with abnormal centrosomes was calculated in U2OS T-REx cells or in two clones of the Nek2A-K37R-myc-His cell line treated with (+) or without (–) doxycycline for 24 h. (B and C) Representative digital deconvolution microscopy images of normal (a) or abnormal (b–d) centrosomes in cells induced to express Nek2A-K37R-myc-His for 24 h before processing for immunofluorescence microscopy with α-Nek2 (B) or anti-γ-tubulin (C) antibodies. In some cells, the centrosome look like a tight cluster of more than two dots (b and c); in other cells, abnormal centrosomes look like a single aggregate that is significantly larger than a normal centrosome (d). (D) Nek2A-K37R-myc-His cells were induced for 24 h before detergent extraction, fixation, and immunostaining with antibodies against γ-tubulin (a and d) and the centriolar marker GT335 (b and e). In cells containing clusters of centrosomes, each dot seems to contain a single centriole. Merged images (c and f) are shown of γ-tubulin (green) and GT335 (red). Bars (B–D), 2 μm.
Where more than two dots were observed, it was important to determine whether each dot contained a centriole rather than simply being an aggregate of centrosomal proteins. Induced cells were therefore stained with the GT335 monoclonal antibody that recognizes polyglutamylated tubulin and hence acts as a well-characterized marker of centrioles (Bobinnec et al., 1998). Whereas only two GT335 dots were clearly distinguished in uninduced cells (Figure 5D, a–c), more than two GT335-stained dots were evident in many cells expressing kinase-dead Nek2A (Figure 5D, d–f). Hence, cells expressing kinase-dead Nek2A accumulate abnormal centrosomes that fall into two categories: centrosomes with extracentrosomal material and centrosomes with supernumerary centrioles that remain in proximity. These two categories are not mutually exclusive making it difficult to score each phenotype separately.
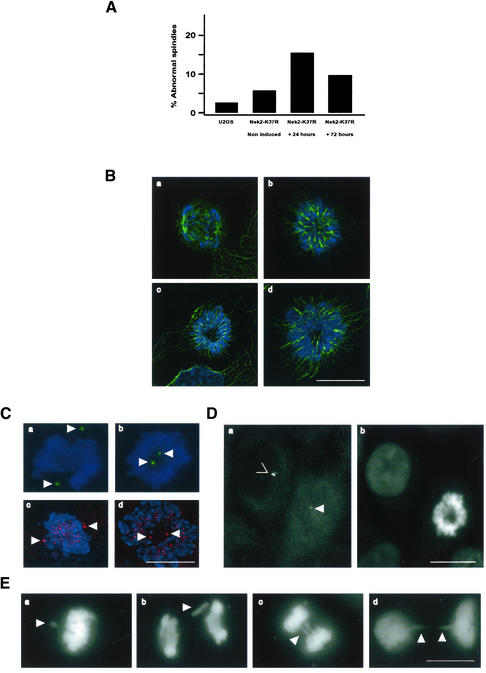
Spindle and Segregation Defects Induced by Kinase-Dead Nek2A Expression
The presence of some cells with more than two centrioles raised the possibility that kinase-dead Nek2A was acting in a dominant-negative manner to prevent separation of mother and daughter centrioles at mitosis. To determine whether such abnormalities may result from a failure of centrosome disjunction, mitotic cells were examined. In the parental cell line, only 3.5% (n = 300) of mitotic cells exhibited any unusual spindle structures with these being a mix of monopolar and multipolar arrays. However, induction of kinase-dead Nek2A for 24 h led to a 4.5-fold increase in abnormal spindles (15.7%; n = 450) (Figure 6A). DNA and microtubule staining revealed that the overwhelming majority of these abnormal mitoses contained monoastral arrays of microtubules surrounded by a chromosome rosette (Figure 6B, b–d). By immunostaining cells with γ-tubulin antibodies and capturing optical z-sections through the monopolar spindles, it was clear that centrosomes had not substantially separated in either the x, y, or z-axis (Figure 6C, b). Measurements showed the average pole-to-pole distance in the monopolar spindles to be 2.1 μm, compared with 9.2 μm in a bipolar metaphase spindle. The frequency of spindle abnormalities did not increase with longer times of induction, suggesting that the maximal level of interference had been achieved for this cell line.
Figure 6.
Mitotic spindle and chromosome segregation defects in Nek2A-K37R cells. (A) Frequency of abnormal (monopolar or multipolar) spindles in mitotic cells was counted in U2OS T-REx cells or in Nek2A-K37R-myc-His cells with (+) or without (–) doxycycline induction for 24 and 72 h. (B) Digital deconvolution microscopy of spindles in Nek2A-K37R-myc-His mitotic cells stained for DNA (blue) and microtubules (green) comparing normal (a) with monopolar (b–d) spindles. (C) Bipolar (a and c) and monopolar (b and d) spindles stained for DNA (blue) and γ-tubulin (a and b; green) or Plk1 (c and d; red) in Nek2A-K37R-myc-His cells. Both Plk1 and γ-tubulin are recruited to poles of monopolar as well as bipolar spindles (arrowheads). Plk1 is also present on kinetochores. (D) C-Nap1 (a) and DNA (b) staining of an adjacent pair of interphase and mitotic cells expressing Nek2A-K37R-myc-His protein. Note that C-Nap1 is significantly less abundant at poles of a monopolar spindle (arrowhead) compared with at interphase centrosomes (>). (E) DNA staining of Nek2A-K37R-myc-His mitotic cells showing clear evidence of missegregation defects (arrowheads) with unaligned chromosomes in metaphase (a), lagging chromosomes in anaphase (b and c), and thin chromatin bridges in telophase (d). Bars (B–E), 15 μm.
Similar monopolar spindle defects have been described under conditions where centrosome maturation, rather than disjunction, is defective (Lane and Nigg, 1996). We therefore immunostained cells for Plk1, which is first recruited to centrosomes during centrosome maturation (Golsteyn et al., 1995). In kinase-dead Nek2A-expressing cells, the recruitment of Plk1 to the poles of monopolar spindles was similar to that in normal bipolar spindles (Figure 6C, c and d). This, together with the normal increase in γ-tubulin abundance (Figure 6C, a and b), indicates that the monopolar spindle phenotype induced by kinase-dead Nek2A is not a result of defective centrosome maturation, at least with respect to Plk1 and γ-tubulin recruitment. Importantly, the abundance of C-Nap1 at poles of monopolar spindles was indistinguishable from that at poles of bipolar spindles, indicating that apparently complete C-Nap1 displacement had occurred (compare Figure 6D with Figure 4A). Thus, the block to centrosome separation could not be attributed to a failure to displace C-Nap1.
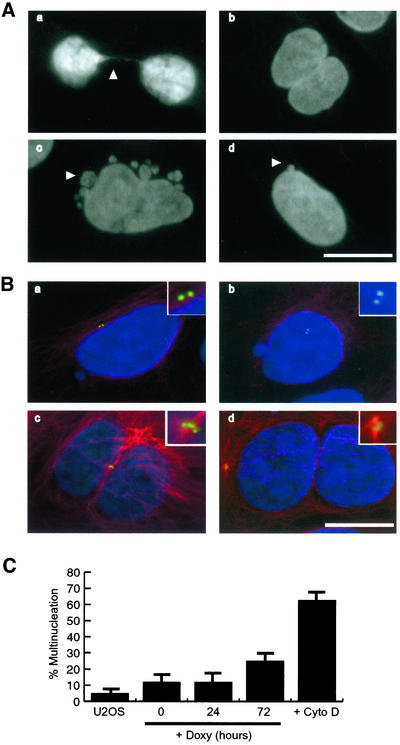
Clearly, a significant proportion of cells expressing kinase-dead Nek2A was capable of forming bipolar spindles, possibly because the recombinant protein was targeted for proteasomal degradation upon mitotic entry. However, chromosome segregation defects were frequently observed in many of these cells as indicated by misaligned chromosomes in metaphase cells, lagging chromosomes in anaphase cells and thin chromatin bridges in telophase cells (Figure 6E). Further evidence for mitotic progression defects came from the appearance of multi- and micronucleated interphase cells (Figure 7A). Immunofluorescence staining of these cells with anti-C-Nap1 antibodies indicated that micronucleated cells generally contained a normal complement of two centrosomes (Figure 7B, a and b), whereas multinucleated cells usually contained clusters of more than two centrosomes suggestive of cytokinesis failure (Figure 7B, c and d). Induction for 72 h of kinase-dead Nek2A caused an approximate sixfold increase in multinucleation compared with the parental U2OS cells (Figure 7C). As a positive control, multinucleation was demonstrated in cells treated with cytochalasin D, which blocks cytokinesis through inhibiting formation of the contractile actin ring. Together, these data provide the first evidence that alteration of Nek2 activity can interfere with mitotic progression in human cells.
Figure 7.
Increased multinucleation in Nek2A-K37R interphase cells. (A) DNA staining of Nek2A-K37R-myc-His interphase cells after 24-h induction with doxycycline showing cells still attached through thin chromatin bridges (a), multinucleated cells (b), and micronucleated cells (c and d). Bar, 15 μm. (B) Immunofluorescence microscopy of micronucleated (a and b) and multinucleated (c and d) Nek2A-K37R-myc-His cells stained for centrosomes (C-Nap1, green), microtubules (α-tubulin, red), and DNA (blue), showing that multinucleated cells frequently contain multiple centrosomes suggestive of defects in cytokinesis. Bar, 15 μm. (C) Percentage of Nek2A-K37R-myc-His cells containing multinucleated cells after 0, 24, and 72 h of doxycycline induction compared with the frequency of multinucleation in parental U2OS cells that were either untreated (U2OS) or treated for 24 h with cytochalasin D (Cyto D).
Expression of Kinase-Dead Nek2A Alters the DNA Content of the Cell Population
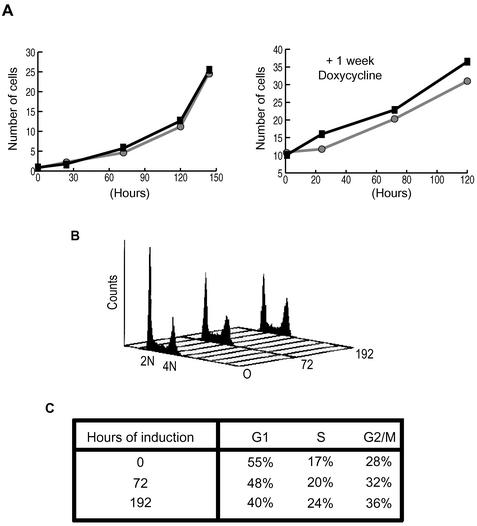
To determine whether these centrosome and mitotic defects led to a major loss of cell viability, cell cycle arrest, or apoptosis, a number of further experiments were performed with the Nek2A-K37R-myc-His cells. Growth curves revealed no significant change in proliferation or survival rate after 12 days of tetracycline induction (Figure 8A). Equally, there was no sudden appearance of apoptotic cells in the population as measured by antibody staining for cleaved cytokeratin 18 (our unpublished data). Similarly, mitotic index counts and Western blotting for the mitosis-specific phospho-H3 epitope revealed no substantial mitotic arrest (our unpublished data). Together, these observations indicate that the centrosome and mitotic abnormalities do not lead to a gross loss of viability within the cell population. Flow cytometric analysis, however, revealed a clear reduction in the proportion of cells with a 2N DNA content after 8-days induction, leading to the majority of cells having >2N DNA (Figure 8, B and C). One interpretation of this result is that there is a delay to cell cycle progression, an intriguing possibility in light of the mutant phenotypes of fungal NIMA-related kinases. However, our data is equally consistent with the increasing percentage of multinucleated cells that were present in the population as a result of either failed mitosis or abortive cytokinesis. Indeed, because the kinase-dead Nek2A cells were derived from an osteosarcoma cancer cell line, it is entirely plausible that they can tolerate a chromosomal instability phenotype that overtime leads to a gradual increase in ploidy without obvious cell death.
Figure 8.
Induction of kinase-dead Nek2A leads to an altered cell cycle distribution. (A) Counts (× 105) of Nek2A-K37R-myc-His cells in the absence (black line, ▪) or presence (grey line, •) of doxycycline. In the left-hand graph, doxycycline was added at T = 0, whereas in the right-hand graph, cells had been grown in the presence of doxycycline for 1 wk before T = 0. (B) Flow cytometry profiles for Nek2A-K37R-myc-His cells induced with tetracycline for 0, 72, and 192 h. The positions of 2N and 4N DNA are indicated. (C) Percentage of cells in each phase of the cell cycle was calculated from the flow cytometry profiles shown in B.
DISCUSSION
In this study, we have examined Nek2 kinase function through generation and phenotypic characterization of tetracycline-inducible cell lines. First, we demonstrate that Nek2A kinase localizes to centrosomes in live cells. Second, we show that inappropriate activation of Nek2A kinase leads to sustained centrosome splitting and the apparent loss of an intercentriolar connection. Third, we reveal that C-Nap1 is still present on split centrosomes, arguing against C-Nap1 displacement being the sole cause of centrosome splitting. Finally, we present evidence that kinase-inactive Nek2A can act in a dominant-negative manner to interfere with bipolar spindle formation and chromosome segregation. Thus, interference with Nek2A activity, both in a positive and negative manner, produces phenotypes that are consistent with a role for this kinase in centrosome disjunction. Importantly, this study provides the first direct evidence that abrogation of Nek2 function has the potential to cause aneuploidy in human cells. These phenotypes also strengthen the hypothesis that centrosome abnormalities can contribute to the sort of chromosome instability that is typical of high-grade tumors (Lingle et al., 1998; Pihan et al., 1998).
Inducible Cell Lines: Novel Tools to Study Nek2 Function In Vivo
Research on Nek2, and its most closely related counterparts in other species, is providing accumulating evidence that this is an important family of cell cycle regulators (reviewed in Fry, 2002). However, the precise functions of Nek2 in mitotic progression remain far from understood. The generation of inducible cell lines provides us with powerful new tools to study the regulation and function of the Nek2A splice variant. Interestingly, transient overexpression of both active and inactive Nek2A kinase led to dispersal of the centrosome and loss of a functional microtubule organizing center (Fry et al., 1998a). This was not a major phenotype of the inducible cell lines, most likely because the level of overexpression per cell is considerably lower than in the transient transfection experiments. Nevertheless, during our live cell recordings of both wild-type and kinase-dead GFP-Nek2A, we did observe centrosomes that seemed to shed fragments or particles into the cytoplasm (our unpublished data). This not only supports the hypothesis that Nek2 contributes to the assembly and maintenance of an intact centrosome structure (Fry et al., 2000b; Uto and Sagata, 2000) but also implies that tumor cells with significant amplification of Nek2 might lose a focused microtubule organizing center.
As a means to unravel Nek2 function, these cell lines rely on protein overexpression. The identification of a specific Nek2 inhibitor would therefore be of great benefit in confirming the role of endogenous Nek2 kinase activity in the control of chromosome segregation and mitotic progression, particularly because it has not yet proven possible to identify effective siRNA molecules despite much effort on our part. Because more than half the cells expressing active GFP-Nek2A have split centrosomes and the GFP-Nek2A protein acts as a vital marker of the centrosome, these cell lines make excellent reagents for high-throughput microscope-based assays to screen for in vivo inhibitors of Nek2 kinase. A small molecule inhibitor may also prove useful as a therapeutic agent in tumors that show up-regulation of Nek2 expression (Wai et al., 2002).
Nek2A Activation Stimulates Centrosome Disjunction
There is growing evidence that centrosome disjunction is triggered by altering the protein phosphorylation status of core centrosomal components. Kinases implicated in regulating centrosome cohesion include Nek2A (Fry et al., 1998a; Meraldi and Nigg, 2001), Cdk2 (Lacey et al., 1999; Meraldi and Nigg, 2001), and p160ROCK (Chevrier et al., 2002), whereas phosphatase involvement has been proposed for PP1 (Helps et al., 2000; Eto et al., 2002) and Cdc14A (Mailand et al., 2002). Nek2A directly binds PP1 via a KVHF motif in its C-terminal noncatalytic domain. Because Nek2A is activated by autophosphorylation (Fry et al., 1999), PP1 can negatively regulate Nek2A by dephosphorylation. Conversely, Nek2A can phosphorylate PP1 reducing its phosphatase activity (Helps et al., 2000). This double-negative feedback produces an exquisitely sensitive bistable switch that dictates the phosphorylation status of Nek2A substrates. In these experiments, we show that increasing the level of Nek2A expression above fivefold is sufficient to flip the switch in favor of centrosome splitting. Moreover, our time-lapse observations of centrosome dynamics suggest that the intercentriolar linkage has been permanently lost. Centrosomes that were >5 μm apart at the start of filming rarely returned to within this distance over the observation period. Clearly, in these in vivo experiments, it is difficult to state with absolute certainty that connections have been irrevocably broken. It remains possible that Nek2A activity alters the conformation of the linkage such that it becomes sufficiently long and flexible to accommodate greater intercentriolar separations. Because our experiments were not performed on synchronized cells, the duplication state of the split centrioles was not known. However, in all our fixed and live cell recordings, four separated structures were never observed strongly implying that Nek2A expression does not trigger the release of procentrioles from their sites of synthesis adjacent to mother and daughter centrioles. We believe that it acts specifically on the connections between mother and daughter centriole.
Kinase-Dead Nek2A Interferes with Centrosome Disjunction and Mitotic Progression
The most striking results of this study are the consequences of overexpressing kinase-dead Nek2A. First, many cells accumulated unusually large and irregularly shaped centrosomes, and centrosomes that clearly had supernumerary (>2) centrioles. Both of these phenotypes are common in cancer cells (Nigg, 2002). The unusually large centrosomes may result from accumulation of excess kinase-dead Nek2A protein, which in turn causes recruitment of other centrosomal proteins, including at least γ-tubulin and C-Nap1. This is consistent with data showing that Xenopus Nek2 is required for recruitment of γ-tubulin during zygotic centrosome assembly (Fry et al., 2000b). Surprisingly, in transient transfection experiments, the opposite phenotype was observed in that expression of kinase-dead Nek2A led to centrosome dispersal. We interpret this difference in terms of the relative expression levels of the ectopic protein: in the induced cell line, the level of overexpression is sufficiently low that most protein can still localize to the centrosome where it acts as a site of recruitment for other centrosomal proteins; in the transient transfections, however, the massive overexpression means that the bulk of the protein can no longer reside at the centrosome and therefore titrates other centrosomal proteins out into the cytoplasm. From either perspective, the experiments emphasize the importance of Nek2 as a critical scaffold element of the centrosome.
Perhaps more informative with regard to Nek2 kinase function is the presence of cells with multiple centrioles. Interpretation of this phenotype is aided by the observation of mitotic cells with monopolar spindles and unseparated centrosomes. Because these centrosomes had recruited both γ-tubulin and Plk1, it seems reasonable to propose that they had undergone some degree of G2/M maturation but then failed to separate. It is interesting to note that centrosomes at the heart of the monoastral microtubule arrays were separated on average by 2.1 μm. For comparison, inhibition of the motor protein Eg5, either by antibody microinjection or by the small molecule inhibitor monastrol, led to monopolar spindles with centrosomes that were <2 μm apart (Blangy et al., 1995; Kapoor et al., 2000). Although it is difficult to know whether this difference is significant based upon the fact that these experiments have been performed in different cell types, it is tempting to speculate that, in the kinase-dead Nek2A-expressing cells, motor proteins have attempted to separate centrosomes but failed because they are still physically attached.
Because we have not been able to follow live cells through mitosis, it is not clear whether the monopolar spindles are eventually resolved into pseudobipolar spindles that can complete mitosis. This may happen in some cells after destruction of the recombinant Nek2A protein. However, the frequent observation of chromosome segregation errors on bipolar spindles as well as multinucleated and micronucleated interphase cells suggests that aneuploidy frequently results from the expression of kinase-dead Nek2A. Unseparated centrosomes may also directly interfere with cytokinesis, leading to tetraploidization. This would provide an explanation for the multinucleated cells with multiple centrosomes. Tetraploidization as a result of mitotic failure has been proposed to account for the supernumerary centrosomes generated by aberrant expression of another centrosomal kinase, Aurora-A (Meraldi et al., 2002). Indeed, this may represent a common pathway for cancer cells to acquire multiple centrosomes and a chromosome instability phenotype, particularly in cells lacking p53 (Nigg, 2002).
The gradual change in ploidy of the whole cell population also supports the idea that kinase-dead Nek2A promotes chromosome instability. Besides this, though, there was no significant decrease in cell proliferation, no striking mitotic arrest and no induction of programmed cell death. Although this was disappointing, similar results have been reported after spindle disruption and aneuploidy induced by interference with another centrosomal protein, pericentrin (Purohit et al., 1999), and it may reflect loss of or adaptation to mitotic checkpoints in transformed cells. Furthermore, proteasomal destruction of Nek2A, as previously discussed, may be an important explanation for the long-term survival, as well as why a larger fraction of mitotic cells do not have abnormal spindles. We are currently generating cell lines expressing nondestructible, kinase-dead Nek2A in an attempt to resolve this issue.
C-Nap1 and the Molecular Nature of the Intercentriolar Linkage
A key substrate for Nek2 at the centrosome is C-Nap1, a large coiled-coil protein with no obvious enzymatic activity (Fry et al., 1998b; Mack et al., 1998). C-Nap1 localizes to proximal ends of centrioles in interphase but is displaced from mitotic spindle poles. This coincides with the time when it is maximally phosphorylated in vivo (Mayor et al., 2002). Using quantitative immunofluorescence microscopy, however, we found that although there is a 10-fold reduction in C-Nap1 abundance at mitotic spindle poles, there is only a twofold reduction in C-Nap1 abundance at split interphase centrioles. Furthermore, C-Nap1 was virtually absent from the poles of monoastral spindles in cells expressing kinase-inactive Nek2A. Our results therefore raise the possibility that Nek2A activation alone is not sufficient to completely trigger C-Nap1 displacement and nor is C-Nap1 displacement necessarily sufficient for centrosome disjunction. Cohen and coworkers estimated that in vitro Nek2A was capable of introducing 13 moles of phosphate into 1 mole of the C-terminal domain of C-Nap1 alone (Helps et al., 2000). Hence, it is possible that during interphase, due to the antagonistic action of PP1, there is only partial phosphorylation of C-Nap1 by the recombinant Nek2A and hence partial displacement. At the G2/M transition full activation of Nek2A would lead to hyperphosphorylation and complete displacement of C-Nap1. Alternatively, other mitotic kinases such as Plk1, Cdk1 or Aurora-A might contribute to the full displacement of C-Nap1 from spindle poles at the G2/M transition (Figure 9). Whether the twofold reduction in C-Nap1 at the centrosome is sufficient to cause disassembly of the intercentriolar linkage is a difficult question to answer. Phosphorylation of C-Nap1 may also cause loss of interaction with other components of the intercentriolar linkage leading to centriole splitting. However, C-Nap1 may not be the only target of Nek2 within the linkage and it could be the cumulative phosphorylation of several proteins by Nek2A that triggers centrosome disjunction. Further insight into the mechanism of centrosome disjunction will clearly require a more detailed understanding of the molecular nature of the intercentriolar linkage.
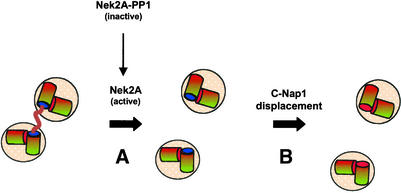
Figure 9.
Centrosome disjunction and C-Nap1 displacement may be distinct events. The schematic model illustrates centrosome disjunction (A) and C-Nap1 displacement (B) as independent events that take place upon onset of mitosis. Disjunction of centrosomes, i.e., loss of cohesion between mother and daughter centrioles, is triggered by activation of Nek2A kinase. This itself is the result of a loss of the antagonistic activity of PP1, perhaps through phosphorylation of PP1 or binding of Inhibitor-2. The substrates of Nek2 involved in centrosome cohesion are likely to include C-Nap1 (blue sphere), although phosphorylation by Nek2 alone may not be sufficient to completely displace C-Nap1 from the centrosome. Nek2 may also have other substrates within the intercentriolar linkage. Likewise, full C-Nap1 displacement may require additional phosphorylation by other mitotic kinases.
Acknowledgments
We thank all members of the laboratory for useful discussion, P. Herron for help with cell culture, and Dr. R.S. Hames for critical reading of the manuscript. We are also grateful to Dr. B. Eddé (Montpellier, France) for the GT335 monoclonal antibody. This work was primarily supported by a grant to A.M.F. from Cancer Research UK. Our laboratory is also supported by grants from The Wellcome Trust and the Association for International Cancer Research. A.M.F. is a Lister Institute Research Fellow.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–02–0108. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-02-0108.
Abbreviations used: Nek2, NIMA-related kinase 2; NIMA, never in mitosis A; C-Nap1, centrosomal Nek2-associated protein 1; PP1, protein phosphatase 1; PCM, pericentriolar material.
References
- Blangy, A., Lane, H.A., d'Herin, P., Harper, M., Kress, M., and Nigg, E.A. (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Bobinnec, Y., Moudjou, M., Fouquet, J.P., Desbruyeres, E., Edde, B., and Bornens, M. (1998). Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskeleton 39, 223–232. [DOI] [PubMed] [Google Scholar]
- Bornens, M., and Moudjou, M. (1999). Studying the composition and function of centrosomes in vertebrates. Methods Cell Biol. 61, 13–34. [DOI] [PubMed] [Google Scholar]
- Bornens, M., Paintrand, M., Berges, J., Marty, M.C., and Karsenti, E. (1987). Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskeleton 8, 238–249. [DOI] [PubMed] [Google Scholar]
- Chevrier, V., Piel, M., Collomb, N., Saoudi, Y., Frank, R., Paintrand, M., Narumiya, S., Bornens, M., and Job, D. (2002). The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J. Cell Biol. 157, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey, S. (2001). Re-evaluating centrosome function. Nat. Rev Mol. Cell. Biol. 2, 688–698. [DOI] [PubMed] [Google Scholar]
- Eto, M., Elliot, E., Prickett, T.D., and Brautigan, D.L. (2002). Inhibitor-2 regulates protein phosphatase-1 complexed with NimA-related kinase to induce centrosome separation. J. Biol. Chem. 277, 44013–44020. [DOI] [PubMed] [Google Scholar]
- Fry, A.M. (2002). The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 21, 6184–6194. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Arnaud, L., and Nigg, E.A. (1999). Activity of the human centrosomal kinase, Nek2, depends upon an unusual leucine zipper dimerization motif. J. Biol. Chem. 274, 16304–16310. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Descombes, P., Twomey, C., Bacchieri, R., and Nigg, E.A. (2000b). The NIMA-related kinase X-Nek2B is required for efficient assembly of the zygotic centrosome in Xenopus laevis. J. Cell Sci. 113, 1973–1984. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., and Faragher, A.J. (2001). Identification of centrosome kinases. Methods Cell Biol. 67, 305–323. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Mayor, T., Meraldi, P., Stierhof, Y.-D., Tanaka, K., and Nigg, E.A. (1998b). C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141, 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, A.M., Meraldi, P., and Nigg, E.A. (1998a). A centrosomal function for the human Nek2 protein kinase, a member of the NIMA-family of cell cycle regulators. EMBO J. 17, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, A.M., Schultz, S.J., Bartek, J., and Nigg, E.A. (1995). Substrate specificity and cell cycle regulation of the Nek2 protein kinase, a potential human homolog of the mitotic regulator NIMA of Aspergillus nidulans. J. Biol. Chem. 270, 12899–12905. [DOI] [PubMed] [Google Scholar]
- Golsteyn, R.M., Mundt, K.E., Fry, A.M., and Nigg, E.A. (1995). Cell cycle regulation of the activity and subcellular localization of PLK1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129, 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames, R.S., and Fry, A.M. (2002). Alternative splice variants of the human centrosomal kinase Nek2 exhibit distinct patterns of expression in mitosis. Biochem. J. 361, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames, R.S., Wattam, S.L., Yamano, H., Bacchieri, R., and Fry, A.M. (2001). APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 20, 7117–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps, N.R., Luo, X., Barker, H.M., and Cohen, P.T.W. (2000). NIMA-related kinase 2 (Nek2), a cell cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem. J. 349, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., and Sluder, G. (2001). “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 15, 1167–1181. [DOI] [PubMed] [Google Scholar]
- Kapoor, T.M., Mayer, T.U., Coughlin, M.L., and Mitchison, T.J. (2000). Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C.L. (1999). The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.H., Choi, J.Y., Jeong, Y., Wolgemuth, D.J., and Rhee, K. (2002). Nek2 localizes to multiple sites in mitotic cells, suggesting its involvement in multiple cellular functions during the cell cycle. Biochem. Biophys. Res. Commun. 290, 730–736. [DOI] [PubMed] [Google Scholar]
- Lacey, K.R., Jackson, P.K., and Stearns, T. (1999). Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA 96, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, H.A., and Nigg, E.A. (1996). Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle, W.L., Lutz, W.H., Ingle, J.N., Maihle, N.J., and Salisbury, J.L. (1998). Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA 95, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, G.J., Rees, J., Sandblom, O., Balczon, R., Fritzler, M.J., and Rattner, J.B. (1998). Autoantibodies to a group of centrosomal proteins in human autoimmune sera reactive with the centrosome. Arthritis Rheum. 41, 551–558. [DOI] [PubMed] [Google Scholar]
- MacRae, T.H., Lange, B.M., and Gull, K. (1990). Production and characterization of monoclonal antibodies to the mammalian sperm cytoskeleton. Mol. Reprod. Dev. 25, 384–392. [DOI] [PubMed] [Google Scholar]
- Mailand, N., Lukas, C., Kaiser, B.K., Jackson, P.K., Bartek, J., and Lukas, J. (2002). Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat. Cell Biol. 4, 317–322. [DOI] [PubMed] [Google Scholar]
- Marshall, W.F. (2001). Centrioles take center stage. Curr. Biol. 11, R487–R496. [DOI] [PubMed] [Google Scholar]
- Mayor, T., Hacker, U., Stierhof, Y.-D., and Nigg, E.A. (2002). The mechanism regulating dissociation of the centrosomal protein C-Nap1 from mitotic spindle poles. J. Cell Sci. 115, 3275–3284. [DOI] [PubMed] [Google Scholar]
- Mayor, T., Meraldi, P., Stierhof, Y.-D., Nigg, E.A., and Fry, A.M. (1999). Protein kinases in control of the centrosome cycle. FEBS Lett. 452, 92–95. [DOI] [PubMed] [Google Scholar]
- Mayor, T., Tanaka, K., Stierhof, Y.-D., Fry, A.M., and Nigg, E.A. (2000). The centrosomal protein C-Nap1 displays properties supporting a role in cell cycle-regulated centrosome cohesion. J. Cell Biol. 151, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E.A. (2002). Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53–/– cells. EMBO J. 21, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., and Nigg, E.A. (2001). Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J. Cell Sci. 114, 3749–3757. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., and Nigg, E.A. (2002). The centrosome cycle. FEBS Lett. 521, 9–13. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A. (2001). Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell. Biol. 2, 21–32. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A. (2002). Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer 2, 815–825. [DOI] [PubMed] [Google Scholar]
- Paintrand, M., Moudjou, M., Delacroix, H., and Bornens, M. (1992). Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 108, 107–128. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C.L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behaviour in vertebrate cells. J. Cell Biol. 149, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, M., Nordberg, J., Euteneuer, U., and Bornens, M. (2001). Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550–1553. [DOI] [PubMed] [Google Scholar]
- Pihan, G.A., Purohit, A., Wallace, J., Knecht, H., Woda, B., Quesenberry, P., and Doxsey, S.J. (1998). Centrosome defects and genetic instability in malignant tumors. Cancer Res. 58, 3974–3985. [PubMed] [Google Scholar]
- Preble, A.M., Giddings, T.M., and Dutcher, S.K. (2000). Basal bodies and centrioles: their function and structure. Curr. Top. Dev. Biol. 49, 207–233. [DOI] [PubMed] [Google Scholar]
- Purohit, A., Tynan, S.H., Vallee, R., and Doxsey, S.J. (1999). Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto, K., and Sagata, N. (2000). Nek2B, a novel maternal form of Nek2 kinase, is essential for the assembly or maintenance of centrosomes in early Xenopus embryos. EMBO J. 19, 1816–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai, D.H., Schaefer, K.L., Schramm, A., Korsching, E., Van Valen, F., Ozaki, T., Boecker, W., Schweigerer, L., Dockhorn-Dworniczak, B., and Poremba, C. (2002). Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int. J. Oncol. 20, 441–451. [PubMed] [Google Scholar]