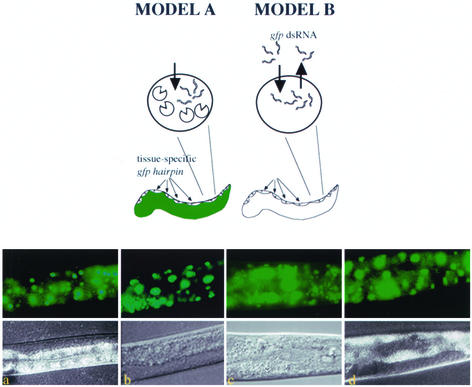
Figure 2.
Assessments of dsRNA cell autonomy. The models depict the experimental design to address the autonomous nature of RNA silencing signals and predicts the RNAi phenotypes of transgenic animals if in vivo-transcribed RNA silencing signals remain cell autonomous (left) or are capable of cellular exit (right). A transgene expressing a gfp hairpin in a tissue-specific manner was introduced into animals expressing a GFP reporter in all cells by standard genetic crosses. Model A predicts that animals would not exhibit systemic silencing for GFP if dsRNA cannot exit the tissue of origin. Cell autonomous RNAi (white) is exhibited as tissue-specific loss of GFP. Model B predicts that animals would exhibit systemic silencing for GFP if RNA silencing signals can exit the cells of origin. (a) The GFP expression pattern in an animal harboring a chromosomally integrated let-858::GFP transgene. (b) A transgene with myo-3::gfp hairpin sequences that delivers dsgfp RNA in muscle (Figure 1, a and c) was crossed into the strain represented in a. (c) A transgene with vit-2::gfp hairpin sequence that delivers dsgfp RNA in gut (Figure 1d) was crossed into animals harboring let-858::GFP. (d) A transgene with unc-119::gfp hairpin sequences that delivers dsgfp RNA in neurons (see MATERIALS AND METHODS) was crossed into animals harboring let-858::GFP. Images were captured using a 40× objective so the overall GFP fluorescence of animals could be compared. RNA silencing was not generally observed in non-hairpin–expressing cells as evidenced by uniform GFP fluorescence in somatic cells.