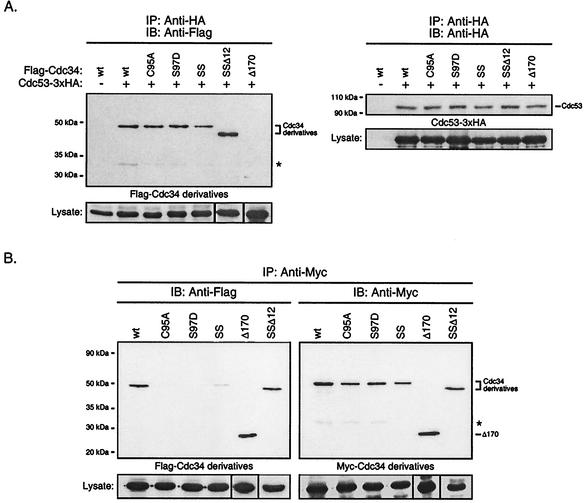
FIG. 4.
(A) Catalytic domain derivatives of Cdc34 interact with Cdc53 of the SCF complex. Total cell extracts were prepared from YPH499 cells coexpressing Cdc53-3xHA and Flag-Cdc34 or one of the Flag-tagged Cdc34 derivatives: C95A, S97D, S73K/S97D (SS), S73K/S97D/Δ12 (SSΔ12), or Δ170. Cdc53-3xHA was immunoprecipitated (IP) with an anti-HA antibody and was detected by immunoblotting (IB) with an anti-HA antibody (right panel). The amount of Flag-Cdc34 or Flag-Cdc34 derivative that coimmunoprecipitated was detected by immunoblotting with an anti-Flag antibody (left panel). The positions of Cdc53, Cdc34, and the Cdc34 derivatives are indicated. The asterisk indicates a proteolytic product of Cdc34. Protein expression levels within the lysates used for immunoprecipitations are shown at the bottoms of the respective panels. (B) Residues within the Cdc34 catalytic domain critical for self-association. The same coimmunoprecipitation strategy as employed for Cdc34 (Fig. 1B) was used for the C95A, S97D, S73K/S97D (SS), and S73K/S97D/Δ12 (SSΔ12) catalytic domain derivatives. Flag- and Myc-tagged versions of these derivatives were expressed in YPH499 cells. Cell lysates were extracted and subjected to immunoprecipitation with anti-Myc antibody followed by immunoblotting with anti-Myc antibody (right panel) and anti-Flag antibody (left panel). The asterisk indicates a proteolytic product of Cdc34. Protein expression levels within the lysates used for immunoprecipitations are shown at the bottoms of the respective panels. wt, wild type.