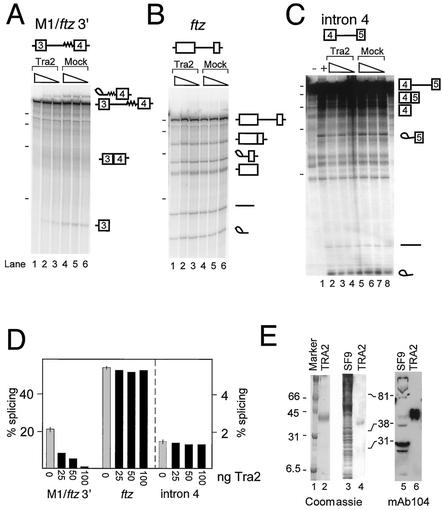
FIG. 2.
Repression of M1/ftz 3′ splicing by recombinant Tra2 protein. In vitro splicing assays were performed with S2 nuclear extracts on the 32P-labeled in vitro-transcribed RNAs depicted above each reaction set. In each set, splicing reactions were carried out in the presence of 100, 50, and 25 ng of recombinant Tra2 protein or in buffer controls without Tra2 (mock) (triangles indicate amounts of recombinant Tra2 and buffer). The positions of reaction intermediates and products are shown schematically at the right. The positions of radiolabeled in vitro-transcribed RNA molecular weight markers run on the same gel (dashes) correspond to 500, 400, 300, and 200 nt from top to bottom. In panel C, the position of the 100-nt band is also indicated. (A) Reactions carried out on M1/ftz 3′. The lariat products comigrate with the pre-mRNA on this gel. (B) Splicing reactions showing that the ftz intron is not repressed by recombinant Tra2. (C) Splicing reactions carried out on inefficiently spliced intron 4 of tra2, which is not regulated by the Tra2 protein in vivo. Lanes 1 and 2, control reactions without and with, respectively, ATP. (D) Quantitation of the splicing reactions shown in panels A to C. Grey bars, average percentages of splicing (products plus intermediates) for the three mock reactions (error bars, ranges of values); black bars, percentages of splicing reaction mixtures containing various amounts of Tra2 protein. Note that the scale for reactions with intron 4, shown to the right of the dashed line, is different from that for M1/ftz and ftz. (E) To demonstrate the degree of purity of the recombinant Tra2 protein used in the above assays, 3.5 μg of Tra2 was loaded onto a SDS-12.5% polyacrylamide gel and stained with Coomassie blue (lane 2). To further test if the purified Tra2 protein was not contaminated with SR proteins, whole-cell lysate from SF9 cells and 1 μg of Tra2 were run in duplicate on separate SDS-12.5% polyacrylamide gels and either stained with Coomassie blue (lanes 3 and 4) or electroblotted and probed with MAb 104 (lanes 5 and 6).