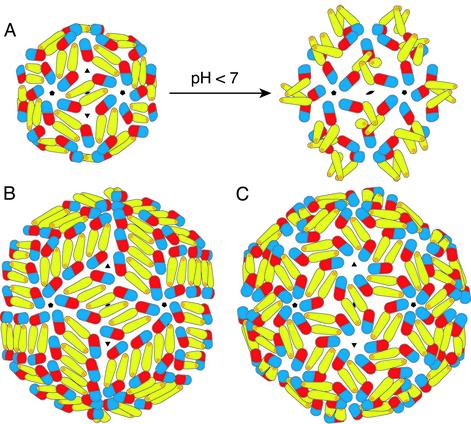
Fig. 4.
Proposed subunit packing interactions in various flaviviral icosahedral assemblies. (A) Suggested transition from the previously studied T = 1 subviral particles (12) to the fusion-competent T = 1 particle at low pH. On acidification, domain II is proposed to swing out about a hinge at the domain I/II interface, creating homotrimeric contacts at the threefold axis. Clusters of three fusion peptides are displayed at the tip of each trimer. (B) The packing in T = 3 virus-like particles deduced from image reconstructions of dengue virions (13). The 180 subunits are not related by local threefold symmetry. (C) Suggested T = 3 packing intermediate for the virion at low pH (13). E is shown in its native (high pH) conformation. Because all monomers are related by local threefold symmetry, the low-pH conformational change will result in the formation of trimers, as in A.