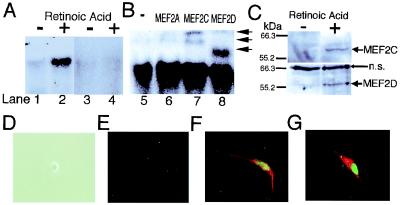
Figure 1.
MEF2 binding activity, protein expression and transfection during neuronal differentiation of P19 cells. (A) Gel shift assays show that MEF2 binding activity increased during neuronal differentiation of P19 cells. A 32P-labeled MEF2 site oligonucleotide was incubated with nuclear extracts from undifferentiated P19 cells (lanes 1 and 3), or from P19 cells treated with retinoic acid for 2 days (lanes 2 and 4). Cold competition by unlabeled MEF2 site oligonucleotides (lanes 3 and 4). (B) Antibody to MEF2A (lane 6), MEF2C (lane 7), or MEF2D (lane 8) was added to the binding mixture for supershift assays. Anti-MEF2C yielded two supershifted bands, representing one or more DNA complexes containing MEF2C, whereas anti-MEF2D produced a single supershifted complex (arrows) (3). (C) Immunoblots revealed that protein expression of MEF2C and MEF2D was induced during neuronal differentiation of P19 cells. Whole-cell lysates from undifferentiated P19 cells or P19 cells treated with retinoic acid for 2 days were used for these immunoblots (n.s., nonspecific bands). (D–G) Overexpression of MEF2C induced a mixed neurogenic/myogenic phenotype. Undifferentiated P19 cells did not display immunoreactivity for MEF2C or neurofilament (D, phase contrast image; E, immunocytochemistry). Undifferentiated P19 cells were transfected with an expression vector for MEF2C. Immunoreactivity for MEF2C is represented in green (E–G), neurofilament in red (E and F), and myosin heavy chain in red (G). Similar findings were observed in 16 experiments in which more than 200 cells were scored.