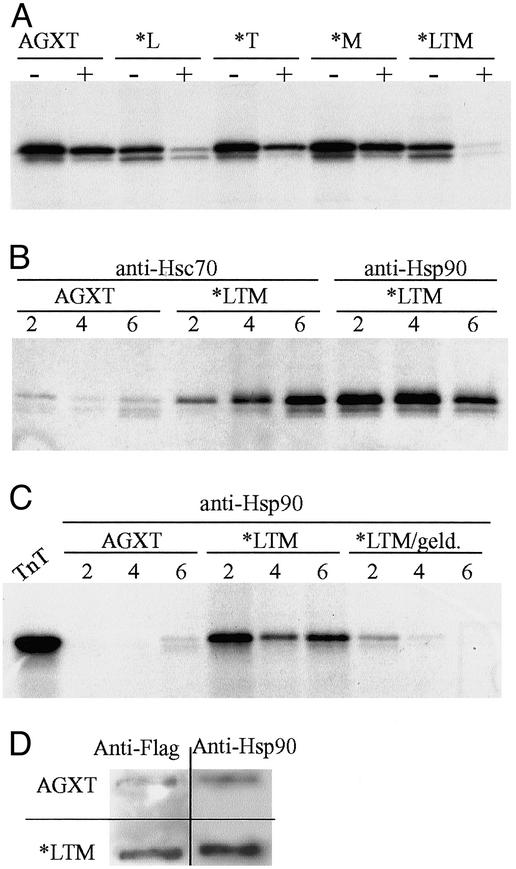
Fig. 2.
(A) SDS/PAGE and fluorography of in vitro-synthesized AGXT proteins subject to 12 μg/ml trypsin for 10 min show significantly higher proteolytic sensitivity for variants carrying the P11L polymorphism, which is potentiated by the coexistence of the I244T mutation. (B) Immunoprecipitation of in vitro-synthesized wild-type AGXT and AGXT*LTM after 2–6 h of incubation at 30°C and reaction with either anti-Hsc70 or anti-Hsp90 antibodies; the mutant allele shows a much more stable association with both molecular chaperones than the wild type, and the association with Hsp90 can be inhibited specifically with 11 μg/ml geldanamycin. (C) The lane TnT represents half the input used for each immunoprecipitation. (D) FRA of nondenaturing lysates from COS7 cells transfected with plasmids containing either the wild-type AGXT or AGXT*LTM cDNA. Duplicate filters probed with either anti-Flag or anti-Hsp90 antibodies show that cells transfected with AGXT*LTM cDNA contain both AGXT and Hsp90 in aggregates retained in the 0.2-μm-pore membrane.