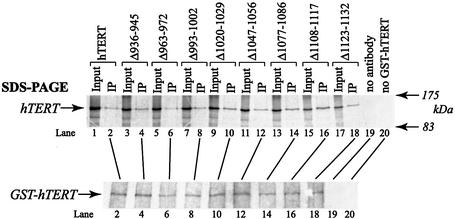
Figure 5.
Physical association of C-terminal hTERT mutants with GST–hTERT in vitro. GST–hTERT and C-terminal mutants were independently synthesized in RRL in the absence of hTR. Immunopurified GST–hTERT was mixed with partially purified [35S]methionine-labeled hTERT or C-terminal mutants. Co-precipitated 35S-labeled hTERT and immunoprecipitated GST–hTERT proteins were detected by SDS–PAGE/autoradiography (top panel) and by western blot analysis (bottom panel), respectively. Five percent of input proteins and 50% of immunoprecipitated proteins were loaded (top panel). Control reactions were performed in the absence of antibody (lane 19) or GST–hTERT (lane 20).