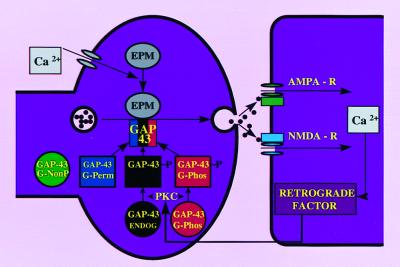
Figure 4.
Proposed mechanism to explain enhanced LTP in transgenic animals overexpressing GAP-43. In nontransgenic animals, presynaptic PKC is activated by an NMDA-dependent postsynaptic retrograde signal. Phosphorylated endogenous GAP-43 (black circle) interacts only with calcium-sensor proteins of the exocytotic protein machine (EPM) (see ref. 49) to enhance release when intraterminal calcium is raised sufficiently. Because low frequency activity does not raise intraterminal calcium to activate EPM sufficiently, phosphorylated GAP-43 alone would be insufficient to induce LTP. Once PKC phosphorylates both endogenous and transgenic G-Phos (red circle), the terminal is “primed” to release more transmitter upon subsequent depolarization of the presynaptic terminal. Because the G-Perm variant of GAP-43 (blue square) can bind to activated EPM without PKC phosphorylation, this mutated form of GAP-43 does not require the influence of NMDA receptor activation as shown in Fig. 2 D and E. Note that either G-Phos or G-Perm transgenic GAP-43, but not the G-NonP variant (green circle), can sum with endogenous GAP-43 to enhance exocytosis.