Abstract
The efficacy of linezolid, alone or in combination with rifampin, against methicillin-susceptible Staphylococcus aureus in rabbits with experimental endocarditis was investigated. Linezolid (50 or 75 mg/kg of body weight), rifampin, and linezolid (25, 50, or 75 mg/kg) plus rifampin produced statistically significant reductions in bacterial counts compared with those in untreated controls. Plasma or valvular vegetation levels of linezolid in the groups treated with the linezolid-rifampin combination were similar to those in the respective linezolid-only treatment groups. At therapeutic levels of linezolid, rifampin resistance was not observed. The results from this experimental model of endocarditis suggest that while rifampin did not provide synergy to the linezolid dosing, it did not antagonize the efficacy of linezolid.
The antimicrobial agent linezolid has been proven to be effective in both experimental and clinical endocarditis, but limited information is available about its use in endocarditis infections when used in combination with other antibacterials (1, 3, 6, 14). The aim of the present study was to investigate the activities of linezolid and rifampin alone or in combination against a clinical Staphylococcus aureus isolate in terms of bactericidal activity, synergy, and emergence of antimicrobial resistance in experimental endocarditis. Although the bactericidal agent rifampin has excellent antistaphylococcal activity, the emergence of rifampin resistance when used as a single agent has limited its use in this manner (19-21). This development of rifampin resistance in infections that are difficult to treat (endocarditis and osteomyelitis) has been documented in many papers and supports the requirement that rifampin should not be administered as a single agent (2, 4, 8, 9, 13). Studies of the combination of rifampin and other antibacterials have reported mixed results, with several studies reporting antagonism or indifference (2, 5, 17, 18). Studies of the rifampin-levofloxacin combination have shown antagonistic results in vitro and in experimental endocarditis (5). In vitro studies of the rifampin-vancomycin combination also have produced antagonistic results (17, 18), but further experimental endocarditis studies of rifampin-vancomycin have reported indifference with this combination (2). In vitro studies of the combination of linezolid and rifampin against several staphylococcal strains using either time-kill curves or the checkerboard method have shown additivity or indifference (11; M. T. Sweeney, K. F. Baldwin, and G. E. Zurenko, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1252, 1999). In vivo studies of the linezolid-rifampin combination in a methicillin-resistant S. aureus bacteremia model in mice also have shown additivity or indifference (10). This is the first in vivo study conducted to assess whether linezolid and rifampin have synergistic, antagonistic, or indifferent effects on efficacy in experimental endocarditis.
The clinical isolate of S. aureus (UC-9258) used in this study has been described previously (14). The MIC tests were performed using a commercial antimicrobial susceptibility panel (MicroScan LZD MIC4; Dade Behring Inc., West Sacramento, Calif.) that included linezolid concentrations of 0.25 to 32 μg/ml. The panels were used for determinations of MICs and minimum bactericidal concentrations (MBCs) in accordance with National Committee for Clinical Laboratory Standards approved standard M7-A5 (12). The MIC of linezolid for this isolate was 2 μg/ml, that of rifampin was ≤0.12 μg/ml, and that of methicillin was 2 μg/ml. The linezolid and rifampin MBCs for UC-9258 were >32 and ≤0.12 μg/ml, respectively.
All procedures in this study were done in compliance with the Animal Welfare Act Regulations (Code of Federal Regulations, parts 1, 2, and 3) and with the Guide for the Care and Use of Laboratory Animals (10a). Left-sided endocarditis was induced in the aortic valves of male New Zealand White rabbits (2 to 2.5 kg) (Covance, Kalamazoo, Mich.) by a previously described catheter method (7). Twenty-four hours after catheter insertion, animals were inoculated via the ear vein with approximately 2.1 × 106 CFU of methicillin-susceptible S. aureus (MSSA) in 1 ml of sterile saline.
Forty-eight hours after bacterial challenge, all animals were randomized into treatment groups as follows. Untreated controls (n = 6) received no drug treatment; linezolid-treated animals received a dose of 25 (n = 4), 50 (n = 7), or 75 (n = 7) mg/kg of body weight orally in a 0.25% methylcellulose vehicle three times daily (t.i.d.) at 8-h intervals; and rifampin-treated animals (n = 7) were given a dose of 5 mg/kg intramuscularly in 20% dimethyl sulfoxide t.i.d. at 8-h intervals. Combination studies were performed with administration of rifampin plus linezolid at a dose of 25 (n = 7), 50 (n = 7), or 75 (n = 8) mg/kg. All antimicrobials were administered for 5 days.
Untreated control animals were sacrificed 48 h after inoculation. Treated animals were sacrificed 8 h after the final dose of linezolid or rifampin by using a 1-ml (200 mg/kg) rapid IV injection of sodium pentobarbital. Blood, aortic valve vegetations, and right kidney samples were collected, homogenized, serially diluted, and plated as previously described (6). Only animals with proper catheter placement completely across the aortic valve were further evaluated; therefore, five rabbits were excluded from the study. The limit of detectable bacteria, in log10 CFU per gram of tissue or milliliter of blood, was determined by calculating the result for one observed bacterial colony in an undiluted sample. The average lower limit of detection for the blood was determined to be 1.3 log10 CFU/ml of blood, that for the valve vegetation was 2.9 log10 CFU/g of tissue, and that for the kidney was 1.8 log10 CFU/g of tissue. Tissue homogenates or blood samples in which no bacterial colonies were detected (culture negative) were assigned the value of one observed colony.
All results are reported as means ± standard deviation (SD). Kruskal-Wallis analysis of variance was used to compare differences between staphylococcal CFU in blood, kidney, and valve vegetation. It was also used to compare differences between plasma levels in the linezolid-only groups versus the linezolid-rifampin combination groups. The Kruskal-Wallis analyses were performed with the Unistat statistical package, version 5. Statistical differences in the culture-negative rates were analyzed via a logit model with the GENMOD procedure in the SAS system for Windows, version 8. A P value of less than 0.05 was considered to be statistically significant.
The mean quantitative blood bacterial culture count from the 48-h untreated controls was 2.88 ± 1.00 log10 CFU/ml. Among the treated rabbits, only the group receiving 25 mg of linezolid/kg (1 of 4 animals) and that receiving rifampin only (1 of 7 animals) had animals with detectible bacteremia at sacrifice. All blood samples from the groups receiving 50 or 75 mg of linezolid/kg and linezolid plus rifampin were culture negative at sacrifice.
Bacterial counts and culture-negative rates for the valve vegetations are shown in Table 1. Valve vegetations in untreated controls had a mean bacterial count of 8.68 ± 0.68 log10 CFU/g, with all six animals having bacteria present in the vegetation. Similar to the results of a previous study of MSSA endocarditis (14), there was a stepwise decrease in the mean bacterial valve counts as the linezolid dose increased. When compared to what was seen with untreated controls, the decrease in valve counts was statistically significant at doses of both 50 and 75 mg/kg. While there were no culture-negative valves in the groups receiving doses of 25 or 50 mg/kg, the group receiving a 75-mg/kg dose had a significantly improved culture-negative rate (3 of 7 animals) compared to the untreated controls. The rifampin and linezolid-plus-rifampin treatment groups all had bacterial levels that were significantly lower than that of the untreated controls.
TABLE 1.
Outcome in valve vegetation and kidney cultures of 5-day treatment of experimental endocarditis caused by MSSA
| Drug(s) and dose (mg/kg)a | No. culture negative/total
|
Mean bacterial count (log10 CFU/g) ± SD
|
||
|---|---|---|---|---|
| Valve | Kidney | Valve | Kidney | |
| No drug | 0/6 | 0/6 | 8.68 ± 0.68 | 4.13 ± 1.39 |
| Linezolid | ||||
| 25 | 0/4 | 0/4 | 8.06 ± 1.04 | 5.10 ± 1.37 |
| 50 | 0/7 | 7/7c | 4.76 ± 1.93c | 1.88 ± 0.12c,d |
| 75 | 3/7c | 5/7c | 3.15 ± 0.56c,d | 2.43 ± 1.57c,d |
| Rifampin | ||||
| 5 | 4/7c | 6/7c | 4.02 ± 2.32c,d | 2.23 ± 0.84c,d |
| Linezolid + rifampinb | ||||
| 25 | 5/7c | 5/7c | 3.44 ± 1.67c,d | 2.00 ± 0.25c,d |
| 50 | 6/7c | 7/7c | 3.44 ± 1.53c,d | 1.91 ± 0.11c,d |
| 75 | 5/8c | 8/8c | 3.47 ± 1.22c,d | 1.82 ± 0.04c,d |
Linezolid was administered orally t.i.d., and rifampin was administered intramuscularly t.i.d.
Doses shown are for linezolid.
P < 0.05 versus results for no-drug group.
P < 0.05 versus results for group receiving 25 mg of linezolid/kg.
In the kidney, all untreated controls had bacteria present (Table 1). While treatment with linezolid at a dose of 25 mg/kg did not significantly reduce the bacterial level in the kidney, significant decreases in mean bacterial counts were seen in the kidneys from animals treated with a linezolid dose of 50 or 75 mg/kg, those treated with rifampin, and all three combination groups.
Blood samples were taken at four time points to determine the linezolid concentration in the plasma (6). The mean linezolid plasma concentrations for the groups receiving doses of 25, 50, and 75 mg/kg with or without rifampin are shown in Table 2. For each dose group, the mean peak linezolid concentrations on days 1 and 5 were above the MIC (MIC of linezolid = 2 μg/ml). The average peak plasma concentration in each group on day 5 showed drug accumulation compared to the day 1 peaks. The day 1 troughs of all linezolid doses were below the MIC for UC-9258; however, at the end of the 5-day dosing period, the groups receiving doses of 50 and 75 mg/kg showed average plasma concentrations above the MIC. When used in combination, rifampin has been reported to accelerate the metabolism of several antimicrobial agents (chloramphenicol, clarithromycin, doxycycline, and fluoroquinolones) by inducing cytochrome P-450 enzymes (15). However, linezolid is not metabolized by P-450 and therefore lower blood levels would not be expected in linezolid-rifampin combinations (16). This effect on linezolid levels was not observed in this study. Plasma concentrations of linezolid in groups receiving doses of 25 and 50 mg/kg were not significantly different from those in the respective linezolid-rifampin combination groups. Plasma linezolid levels in the group receiving 75 mg of linezolid-rifampin/kg were significantly higher than those in the group receiving only linezolid at 75 mg/kg at the day 5 peak. Although the averages were significantly different for these groups, the ranges of plasma levels were 19.8 to 52.3 μg/ml and 33.3 to 51.7 μg/ml for the linezolid-only and linezolid-rifampin groups, respectively. We conclude that except for the low level of linezolid in one rabbit from the linezolid-only group (19.8 μg/ml), the overlap in plasma levels shows that rifampin does not reduce linezolid plasma levels. Valve vegetation concentrations of linezolid were similar in the linezolid-only groups and the respective linezolid-rifampin combination groups. A previous study using the same rifampin dosing regimen (5 mg/kg t.i.d. intramuscularly) reported concentrations of 3.6 ± 0.6 μg/ml, well above the MIC of rifampin against this strain of MSSA (MIC ≤ 0.12 μg/ml) (4).
TABLE 2.
Concentrations of linezolid in plasma and valve vegetation
| Linezolid dose (mg/kg) (no. of animals)c | Mean concn in plasma (μg/ml) ± SD
|
Mean concn in vegetation (μg/g) ± SD at sacrifice on day 6 | |||
|---|---|---|---|---|---|
| Day 1
|
Day 5
|
||||
| Peak (1 h) | Trough (8 h) | Peak (1 h) | Trough (8 h) | ||
| 25 (4) | 4.05 ± 5.82 | 0.13 ± 0.16 | 13.15 ± 6.47 | 0.26 ± 0.39 | 0.65b |
| 50 (7) | 9.28 ± 6.74 | 0.40 ± 0.36 | 30.94 ± 9.53 | 6.18 ± 6.70 | 4.09 ± 3.72 |
| 75 (7) | 12.31 ± 7.01 | 0.40 ± 0.44 | 34.01 ± 10.23 | 13.92 ± 9.76 | 8.73 ± 5.36 |
| 25 + RIF (7) | 7.28 ± 4.55 | 0.07 ± 0.07 | 12.12 ± 5.78 | 1.93 ± 3.24 | 1.88 ± 2.43 |
| 50 + RIF (7) | 14.68 ± 12.02 | 0.13 ± 0.19 | 24.63 ± 9.76 | 6.23 ± 4.22 | 3.97 ± 2.76 |
| 75 + RIF (8) | 15.16 ± 10.66 | 0.92 ± 0.80 | 43.65 ± 6.01a | 20.78 ± 9.93 | 13.04 ± 5.24 |
P ≤ 0.05 for group receiving 75 mg of linezolid/kg versus that receiving 75 mg of linezolid-rifampin/kg.
There was only one vegetation with linezolid levels above the limit of detection in the group receiving 25 mg of linezolid/kg.
RIF, rifampin.
Bacterial colonies recovered from animals treated with linezolid, rifampin, or linezolid plus rifampin were retested for linezolid and rifampin susceptibility by repeating the MIC determinations. There was no change in the MICs of linezolid for any of the bacterial strains recovered from culture-positive valvular vegetations. One bacterial strain from the rifampin therapy group had developed resistance to rifampin (MIC > 2 μg/ml), and one strain from the group receiving rifampin-linezolid at 25 mg/kg had also developed resistance to rifampin. Rifampin resistance was not observed at therapeutic levels of linezolid. While rifampin resistance developed in two rabbits during 5 days of therapy in this study, no recovered isolate had developed resistance to linezolid.
Time-kill studies were performed with peak and trough concentrations of linezolid (20 and 6 μg/ml, respectively) with or without peak levels of rifampin (3 μg/ml). Neither linezolid (6 or 20 μg/ml) nor rifampin (3 μg/ml) reduced bacterial levels more than 1 log10 after 6 h of incubation. The combination of rifampin with either linezolid concentration also did not reduce bacterial levels more than 1 log10 after 6 h of incubation. At 24 h, neither therapy with the individual drugs alone nor combination therapy was bactericidal (<3-log10 reduction for each group). At either time point, antagonism or synergism was not measured in the combination of therapies versus individual treatments. It is interesting that at 48 h, rifampin resistance was observed.
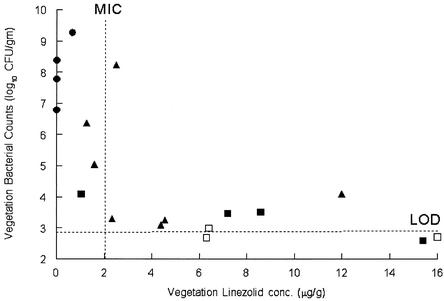
The purpose of this study was to compare the therapeutic efficacies of linezolid and rifampin combination therapy and to monitor antibacterial resistance using an in vivo model of an MSSA infection. In previous studies with linezolid in rabbit methicillin-resistant S. aureus endocarditis, our group documented that a linezolid dose of 50 or 75 mg/kg significantly reduced bacterial vegetation titers and that this was associated with plasma linezolid levels above the MIC at the end of the dosing interval (6). Figure 1 displays data from the present study on the relationship between linezolid levels in the valve vegetations that are above the MIC at the end of the dosing interval and the subsequent reduction in vegetation bacterial counts.
FIG. 1.
Bacterial vegetation counts versus linezolid concentration in the vegetation. The concentration of linezolid in the vegetations and the valve vegetation bacterial counts were determined after 5 days of treatment. The symbols represent linezolid doses of 25 (•), 50 (▴), and 75 (▪) mg/kg. The closed symbols represent culture-positive vegetations, and the open symbols represent culture-negative valvular vegetations (bacterial counts below the limit of detection [LOD]). The linezolid MIC for this strain of MSSA is 2 μg/ml, and the limit of detection is 2.9 to 3.2 log10 CFU/g.
In conclusion, linezolid and rifampin in combination in vivo and in vitro did not antagonize each other and may be of clinical interest in the treatment of infections due to S. aureus. The emergence of rifampin resistance was not detected at therapeutic levels of linezolid. Lowered levels of linezolid in the plasma or valve vegetation were neither predicted for these combination studies nor observed. The documented antagonistic effect of rifampin with other antibacterials was not detected in this study.
Acknowledgments
We acknowledge Ming T. Kuo and Ray Zielinski for expert assistance in measuring plasma linezolid concentrations. We also acknowledge Kathy Justen, Mark Shattuck, and Robert Griffin for assistance with dosing.
REFERENCES
- 1.Babcock, H. M., D. J. Ritchie, E. Christiansen, R. Starlin, R. Little, and S. Stanley. 2001. Successful treatment of vancomycin-resistant Enterococcus endocarditis with oral linezolid. Clin. Infect. Dis. 32:1373-1375. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, A. S., and K. Lam. 1985. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J. Infect. Dis. 151:157-165. [DOI] [PubMed] [Google Scholar]
- 3.Birmingham, M. C., R. R. Craig, B. Hafkin, W. M. Todd, S. M. Flavin, J. D. Root, G. S. Zimmer, D. H. Batts, and J. J. Schentag. 1999. Critical care patients with significant, resistant, gram-positive infections enrolled in the linezolid compassionate use protocol. Crit. Care Med. 27(Suppl. S):42. [Google Scholar]
- 4.Chambers, H. F., M. Kartalija, and M. Sande. 1995. Ampicillin, sulbactam, and rifampin combination treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J. Infect. Dis. 171:897-902. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, H. F., Q. X. Liu, L. L. Chow, and C. Hackbarth. 1999. Efficacy of levofloxacin for experimental aortic-valve endocarditis in rabbits infected with viridans group streptococcus or Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2742-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dailey, C. F., C. L. Dileto-Fang, L. V. Buchanan, M. P. Oramas-Shirey, D. H. Batts, C. W. Ford, and J. K. Gibson. 2001. Efficacy of linezolid in treatment of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin, R., G. Modin, S. Kunz, R. Rich, and M. Sande. 1990. Comparative efficacies of ciprofloxacin, perfloxacin, and vancomycin in combination with rifampin in a rat model of methicillin-resistant Staphylococcus aureus chronic osteomyelitis. Antimicrob. Agents Chemother. 34:1014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eng, R. H., S. M. Smith, M. Tillem, and C. Cherubin. 1985. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch. Intern. Med. 145:146-148. [DOI] [PubMed] [Google Scholar]
- 10.Ford, C. W., J. C. Hamel, D. M. Wilson, J. K. Moerman, D. Stapert, R. J. Yancey, Jr., D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vivo activities of U-100592 and U-10076, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob. Agents Chemother. 40:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy of Sciences, Washington, D.C.
- 11.Mulazimoglu, L., S. D. Drenning, and V. L. Yu. 1996. In vitro activities of two novel oxazolidinones (U100592 and U100766), a new fluoroquinolone (trovafloxacin), and dalfopristin-quinupristin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:2428-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Norden, C. W. 1983. Experimental chronic staphylococcal osteomyelitis in rabbits: treatment with rifampin alone and in combination with other antimicrobial agents. Rev. Infect. Dis. 5:S491-S494. [DOI] [PubMed] [Google Scholar]
- 14.Oramas-Shirey, M. P., L. V. Buchanan, C. L. Dileto-Fang, C. F. Dailey, C. W. Ford, D. H. Batts, and J. K. Gibson. 2001. Efficacy of linezolid in a staphylococcal endocarditis rabbit model. J. Antimicrob. Chemother. 47:349-352. [DOI] [PubMed] [Google Scholar]
- 15.Physicians' Desk Reference. 2002. Rifadin capsules. [Online.] http://www.pdrel.com.
- 16.Slatter, J. G., L. A. Adams, E. C. Bush, K. Chiba, P. T. Daley-Yates, K. L. Feenstra, S. Koike, N. Ozawa, G. W. Peng, J. P. Sams, M. R. Schuette, and S. Yamaki. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907-924. [DOI] [PubMed] [Google Scholar]
- 17.Tofte, R. W., and J. Solliday. 1981. Staphylococcus aureus infection of dialysis shunt: absence of synergy with vancomycin and rifampin. South. Med. J. 74:612-615. [DOI] [PubMed] [Google Scholar]
- 18.Watanakunakorn, C., and J. C. Gueriero. 1981. Interaction between vancomycin and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 19:1089-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehrli, W. 1983. Rifampin: mechanisms of action and resistance. Rev. Infect. Dis. 5:S407-S411. [DOI] [PubMed] [Google Scholar]
- 20.Zavasky, D. M., and M. A. Sande. 1998. Reconsideration of rifampin: a unique drug for a unique infection. JAMA 279:1575-1577. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerli, W., A. F. Widner, M. Blatter, R. Frei, and P. E. Ochsner. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]